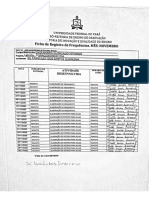
Professional Documents
Culture Documents
Solubilidade
Uploaded by
sil_franciley0 ratings0% found this document useful (0 votes)
9 views1 pagesolubilidade
Original Title
solubilidade
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentsolubilidade
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
9 views1 pageSolubilidade
Uploaded by
sil_francileysolubilidade
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 1
2.6 SOLUBILITY PRODUCT
For sparingly soluble salts (ie. those of which the solubility is less than
0.01 mol per L) it is an experimental fact that the mass action product of the
concentrations of the ions is a constant at constant temperature, This product
K, is termed the ‘solubility product’. For a binary electrolyte:
AB=A*+B™
Kyaw = [A*] x [Bo]
In general, for an electrolyte A,B, which ionises into pA** and gB?~ ions:
A,B, = pA** + qBP-
Kyajay = [AS x [BY]?
A plausible deduction of the solubility product relation is the following. When
excess of a sparingly soluble electrolyte, say silver chloride, is shaken up with
water, some of it passes into solution to form a saturated solution of the salt
and the process appears to cease. The following equilibrium is actually present
(the silver chloride is completely ionised in solution):
AgCl(solid) = Ag* +C1-
The rate of the forward reaction depends only upon the temperature, and at
any given temperature:
rn=ky
where k, is a constant. The rate of the reverse reaction is proportional to the
activity of each of the reactants; hence at any given temperature:
1 = ky x dg: X dg
where k; is another constant. At equilibrium the two rates are equal, ie
ky = ky X agg X dq,
or gg X dey = ki/ky = Kangen
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Ficha Frequencia-AGOSTODocument1 pageFicha Frequencia-AGOSTOsil_francileyNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Relatório NovembroDocument1 pageRelatório Novembrosil_francileyNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Relatório NovembroDocument1 pageRelatório Novembrosil_francileyNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Scheutz 2009 409-455 PDFDocument48 pagesScheutz 2009 409-455 PDFsil_francileyNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Ficha de Frequência NovembroDocument1 pageFicha de Frequência Novembrosil_francileyNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Scheutz 2009 409-455 PDFDocument48 pagesScheutz 2009 409-455 PDFsil_francileyNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Ficha Frequencia-OutubroDocument1 pageFicha Frequencia-Outubrosil_francileyNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Ficha Frequencia-OutubroDocument1 pageFicha Frequencia-Outubrosil_francileyNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Ficha de Frequência NovembroDocument1 pageFicha de Frequência Novembrosil_francileyNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- Relatório NovembroDocument1 pageRelatório Novembrosil_francileyNo ratings yet
- Portaria Normativa Nº 21, de 21 de Dezembro de 2017Document5 pagesPortaria Normativa Nº 21, de 21 de Dezembro de 2017sil_francileyNo ratings yet
- Ficha de Frequência OutubroDocument1 pageFicha de Frequência Outubrosil_francileyNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Tese Camada de Atenuação de Metano - Março 2008 - Final 1Document167 pagesTese Camada de Atenuação de Metano - Março 2008 - Final 1sil_francileyNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Teixeira Processo Oxidacao 2007Document9 pagesTeixeira Processo Oxidacao 2007sil_francileyNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- AletaDocument2 pagesAletasil_francileyNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- 01-Methane 150134 PDFDocument14 pages01-Methane 150134 PDFsil_francileyNo ratings yet
- A10v7n3 PDFDocument6 pagesA10v7n3 PDFsil_francileyNo ratings yet
- MetanoDocument116 pagesMetanoleandro8schultzNo ratings yet
- 44coisas Previdencia PDFDocument17 pages44coisas Previdencia PDFsil_francileyNo ratings yet
- Flexibilização UFPADocument2 pagesFlexibilização UFPAsil_francileyNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Anexos ProjetoDocument76 pagesAnexos Projetosil_francileyNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- 45 Bioplasticos Amido 2 PDFDocument3 pages45 Bioplasticos Amido 2 PDFsil_francileyNo ratings yet
- Educação Do Campo, Agricultura FamiliarDocument10 pagesEducação Do Campo, Agricultura Familiarsil_francileyNo ratings yet
- Regimento Do Instituto de Tecnologia - ITECDocument26 pagesRegimento Do Instituto de Tecnologia - ITECsil_francileyNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- 45 Bioplasticos Amido 2 PDFDocument3 pages45 Bioplasticos Amido 2 PDFsil_francileyNo ratings yet
- DescribingWaterFlow Kosugi 1998Document10 pagesDescribingWaterFlow Kosugi 1998sil_francileyNo ratings yet
- 45 Bioplasticos Amido 2 PDFDocument3 pages45 Bioplasticos Amido 2 PDFsil_francileyNo ratings yet
- Projeto Pedagogico - IEDDocument24 pagesProjeto Pedagogico - IEDsil_francileyNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Gestão em SaudeDocument68 pagesGestão em Saudesil_francileyNo ratings yet
- Comparison of The Tree Hydraulic Property PDFDocument24 pagesComparison of The Tree Hydraulic Property PDFsil_francileyNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)