Professional Documents
Culture Documents
MethyleneBlue Analysis
Uploaded by
ManuelilloCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MethyleneBlue Analysis
Uploaded by
ManuelilloCopyright:
Available Formats
Application
Note: 51867
Analysis of Methylene Blue Reduction
by Ascorbic Acid
Nicole Kreuziger Keppy, Michael W. Allen, Ph.D., Thermo Fisher Scientific, Madison, WI, USA
Introduction
Key Words
Ascorbic Acid
Kinetics
Methylene Blue
Reaction Rate
Reaction
Mechanism
Chemical kinetics is the study of properties of chemical
processes such as reaction rates, mechanisms and transition
states. The mechanism and rate of chemical reactions can
be determined using UV-Visible spectrophotometry. In this
application note, the reaction of methylene blue with
ascorbic acid is monitored with a Thermo Scientific
Evolution Array UV-Visible spectrophotometer. The rate
constant is also determined using Thermo Scientific
VISIONcollect software.
Methylene blue (MB+) is a water-soluble dye molecule.
Under acidic conditions, it is easily reduced to the
colorless hydrogenated molecule leucomethylene blue
(LB+) by ascorbic acid (H2A) as shown in Figure 1. The
stoichiometry of the overall reaction is 1:1 and is
represented in the equation:
MB+ + H2A LB+ + D
UV-Visible
Spectroscopy
Background
Figure 1: Reaction Mechanism of methylene blue with ascorbic acid
The mechanism of a chemical reaction can be identified by
measuring a change in the overall reaction rate caused by
changes in the concentration of individual reactants. In the
simple chemical reaction:
A+BP
the reaction rate (V) is proportional to the concentration
of reactants, such that:
V = k [A][B]
In this rate expression, k is the rate constant, is the
order of the reaction with respect to A, and is the order
of the reaction with respect to B. Thus, the overall order
of the reaction is:
n=+
A rate constant (kexp) is determined experimentally by
measuring the change in concentration of methylene blue
at 665 nm. As a first approximation, the rate of
disappearance of MB+ can be expressed as in Equation 1
below. By strategically varying the individual reactants, a
reaction mechanism can be determined by calculating
constants k0 and k1 in Equation 1 using the experimental
results.1
Equation 1:
d[MB+]
dt
= -{k0 + k1[HCl]}[H2A][MB+]
= -kexp[MB+]
Experiment and Results
Stock solutions of methylene blue (4.0 x 10-4 mol/L),
ascorbic acid (0.5 mol/L) and hydrochloric acid (2.0 mol/L)
were prepared. Fifteen sample solutions were prepared
from these stock solutions according to the conditions
defined in Table 1. A blank was prepared for each sample
by mixing together all of the reagents in the corresponding
sample except methylene blue. All measurements were
performed immediately following the addition of methylene
blue to the sample due to the fast nature of the reaction.
Time-based Kinetics mode was programmed using the
parameters shown in Figure 2.
Sample #
Ascorbic
Acid (M)
HCl (M)
Methylene
Blue (M)
Total Run
Time (s)
Set A: Varying Methylene Blue Concentration [MB+]
A1
A2
A3
A4
A5
0.3
0.3
0.3
0.3
0.3
0.025
0.025
0.025
0.025
0.025
3.5 x 10-5
2.5 x 10-5
1.5 x 10-5
9.0 x 10-6
4.0 x 10-6
200
120
120
120
120
Figure 2: Method parameters for methylene blue reduction analysis
Set B: Varying Ascorbic Acid Concentration [H2A]
B1
B2
B3
B4
B5
0.2
0.2
0.2
0.2
0.2
0.000
0.004
0.008
0.016
0.036
1.5 x 10-5
1.5 x 10-5
1.5 x 10-5
1.5 x 10-5
1.5 x 10-5
600
600
360
180
120
Set C: Varying Hydrochloric Acid Concentration [HCl]
C1
C2
0
0.1
0.045
0.045
1.5 x 10-5
1.5 x 10-5
C3
C4
C5
0.2
0.4
0.5
0.045
0.045
0.045
1.5 x 10-5
1.5 x 10-5
1.5 x 10-5
150
120
120
120
120
Table 1: Sample preparation and experimental conditions for the analysis of
methylene blue reduction
Figure 3: Absorbance decay of methylene blue under condition A1
Sample #
Start Time (s)
End Time (s)
Kexp
A1
A2
A3
A4
A5
0
0
0
0
0
B1
B2
B3
B4
B5
C1
C2
C3
C4
C5
0
0
0
0
0
0
0
0
0
0
80
80
80
80
80
Average
600
450
266
150
70
150
120
90
46
36
0.0616
0.0645
0.0607
0.0631
0.0619
0.0624
0.0000
0.0097
0.0172
0.0333
0.0693
0.0179
0.0454
0.0645
0.1076
0.1310
Table 2: Rate calculation range and experimental rate for each
experimental condition
The Relationship Between Rate Constant and Methylene
Blue Concentration, [MB+] (Set A):
The change in absorbance of methylene blue at 665 nm,
plotted as a function of time, is shown in Figure 3. A first
order fit of the absorbance between 0 and 80 seconds
was used to determine an experimental rate constant (kexp)
of 0.0616. The data collected for the remaining samples
(A2-C5) shows a similar pattern and their rate constants
are shown in Table 2.
One benefit of the Evolution Array is its ability to
collect the entire spectrum during kinetics experiments.
The full spectrum of methylene blue can be observed by
clicking the full spectrum icon in Time Based Kinetics Mode
in the VISIONcollect software. The individual spectra
for sample A1 are shown in Figure 4. These spectra were
also used to construct the decay curve shown in Figure 3.
Figure 5: Sample set B results show kexp as a function of ascorbic acid
concentration
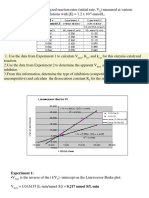
The Relationship Between Rate Constant and HCl
Concentration, [HCl] (Set C):
Figure 4: Spectra of methylene blue reduction under condition A1
In the final set of solutions, the concentration of HCl was
varied according to Set C, Table 1. Plotting kexp against
HCl concentration illustrates the linear relationship of the
rate with respect to the concentration of HCl (Figure 6).
The resulting linear fit has a slope of 0.2212 M-1s-1 and a
R2 value of 0.9981. Taking the slope as the experimental
rate and substituting it into Equation 1 we find:
k1[H2A] = 0.2212 M-1s-1
As shown in Table 2, variations in kexp are small with
respect to methylene blue concentration. The average
value of kexp is approximately 0.0624. All of the measured
values fall within a small range of this average value and
do not exhibit a trend in variation as a result of a decrease
in concentration. Thus, we can conclude that the initial
concentration of methylene blue is not a significant factor
in the rate equation.
Entering the concentration of H2A (0.045 M), the
value for k1 is determined as follows:
k1 = 4.92 M-2s-1
Entering the value of k1 into Equation 2, the value of
k0 is determined as follows:
k0 + (4.92 M-2s-1)(0.2 M) = 1.9026 M-1s-1
k0 = 0.92 M-1s-1
The Relationship Between Rate Constant and Ascorbic
Acid Concentration, [H2A] (Set B):
To determine the affect of ascorbic acid on the overall
rate, the concentration of H2A was varied according to
the conditions defined in Set B, Table 1. A plot of kexp
against H2A concentration exhibits a linear relationship as
shown in Figure 5. A linear fit of the data results in a
slope of 1.9026 M-1s-1 with a R2 value of 0.9983. The
slope of this curve is the kexp and can be entered into
Equation 1:
Equation 1:
k0 + k1[HCl] = 1.9026 M-1s-1
The concentration of HCl in this sample set is held
constant at 0.2 M. Entering this value into the equation
above yields Equation 2:
Equation 2:
k0 + k1(0.2 M) = 1.9026 M-1s-1
Figure 6: Sample set C results show kexp as a function of HCl concentration
Experimental Rate Law
The rate law is obtained by combining the experimental
results from sets A-C. This rate law for the oxidation of
methylene blue by ascorbic acid under acidic conditions is
shown in Equation 3.
Equation 3:
d[MB+]
dt
= -{0.92 + 4.92[HCl]}[H2A][MB+] = -kexp[MB+]
Conclusion
References
In addition to these
The analysis of methylene blue reduction by ascorbic acid
is performed quickly and easily with the Evolution Array
and VISIONcollect software. In this experiment, rate data
was obtained for the analysis and rate constants were
simultaneously calculated using the kinetic analysis function
of the VISIONcollect software. The export function of the
VISIONcollect software facilitates further data processing
in EXCEL. From this analysis it was determined that the
rate constant of the reaction is dependent on the
concentration of both ascorbic acid and HCl and not on
the initial concentration of methylene blue.
1. Mowry, S. and Orgen, P.J. (1999) Kinetics of Methylene Blue Reduction
by Ascorbic Acid, J Chem. Ed. 76(7), 970-974.
offices, Thermo Fisher
Scientific maintains
a network of representative organizations
throughout the world.
Africa-Other
+27 11 570 1840
Australia
+61 2 8844 9500
Austria
+43 1 333 50 34 0
Belgium
+32 53 73 42 41
Canada
+1 800 530 8447
China
+86 10 8419 3588
Denmark
+45 70 23 62 60
Europe-Other
+43 1 333 50 34 0
Finland / Norway /
Sweden
+46 8 556 468 00
France
+33 1 60 92 48 00
Germany
+49 6103 408 1014
India
+91 22 6742 9434
Italy
+39 02 950 591
Japan
+81 45 453 9100
Latin America
+1 608 276 5659
Middle East
+43 1 333 50 34 0
Netherlands
+31 76 579 55 55
South Africa
+27 11 570 1840
Spain
+34 914 845 965
Switzerland
+41 61 716 77 00
UK
+44 1442 233555
USA
+1 800 532 4752
www.thermo.com
2010 Thermo Fisher Scientific Inc. All rights reserved. Excel is a registered trademark of Microsoft Corporation. All other trademarks are the property of Thermo Fisher Scientific Inc. and its
subsidiaries. Specifications, terms and pricing are subject to change. Not all products are available in all countries. Please consult your local sales representative for details.
Thermo Electron Scientific
Instruments LLC, Madison, WI
USA is ISO Certified.
AN51867_E 01/10M
Part of Thermo Fisher Scientific
You might also like
- Methylene Blue/Homeopathy Returns: Viral Loads, Malaria, Wrinkles & Anti-AgingDocument7 pagesMethylene Blue/Homeopathy Returns: Viral Loads, Malaria, Wrinkles & Anti-AgingVIJAY VATSAL100% (3)
- Methylene Blue, An Old Drug With New IndicationsDocument7 pagesMethylene Blue, An Old Drug With New Indicationsmariaclarabaang100% (2)
- Professional Plaquex InfoDocument48 pagesProfessional Plaquex Infoamelia rumenta100% (1)
- Jean Lugol Iodine PDFDocument234 pagesJean Lugol Iodine PDFvavacane100% (1)
- History of EDTA Chelation Therapy-Michael Schachter 9-14-13 With Notes PDFDocument56 pagesHistory of EDTA Chelation Therapy-Michael Schachter 9-14-13 With Notes PDFMiro KasoNo ratings yet
- Binders - What Are They and Why Use Them-5nov'22-FinalDocument5 pagesBinders - What Are They and Why Use Them-5nov'22-Finalvitaolga7No ratings yet
- Dmso Handbook For DoctorsDocument44 pagesDmso Handbook For DoctorsJan Pran100% (4)
- Lightfortherapy BookDocument29 pagesLightfortherapy Bookapi-228757290No ratings yet
- Early History of Methylene BlueDocument6 pagesEarly History of Methylene BlueOdessa File100% (1)
- Hormones: Mark Angelo R. Jacosalem Faye Bernadette O. Reyes Ma. Elizabeth VerteraDocument40 pagesHormones: Mark Angelo R. Jacosalem Faye Bernadette O. Reyes Ma. Elizabeth VerteraGelo Robin JacosalemNo ratings yet
- Methylene BlueDocument30 pagesMethylene Bluesubhaga dubey100% (1)
- Lumbrokinase A Potentand Stable Fibrin Specific Plasminogen ActivatorDocument15 pagesLumbrokinase A Potentand Stable Fibrin Specific Plasminogen ActivatorSyahira Almun100% (1)
- London Cancer Methylene Blue Guideline v1Document5 pagesLondon Cancer Methylene Blue Guideline v1Tan Wei Lin33% (3)
- The Sodium Bicarbonate Cancer Cure - Fraud or Miracle?: Sodium Bicarbonate in the Prevention and Treatment of All Sickness and DiseaseFrom EverandThe Sodium Bicarbonate Cancer Cure - Fraud or Miracle?: Sodium Bicarbonate in the Prevention and Treatment of All Sickness and DiseaseRating: 5 out of 5 stars5/5 (2)
- The Chelation Revolution: Breakthrough Detox Therapy, with a Foreword by Tammy Born Huizenga, D.O., Founder of the Born ClinicFrom EverandThe Chelation Revolution: Breakthrough Detox Therapy, with a Foreword by Tammy Born Huizenga, D.O., Founder of the Born ClinicNo ratings yet
- Oxygen to the Rescue: Oxygen Therapies, and How They Help Overcome Disease and Restore Overall HealthFrom EverandOxygen to the Rescue: Oxygen Therapies, and How They Help Overcome Disease and Restore Overall HealthRating: 4.5 out of 5 stars4.5/5 (3)
- PEMF Machine Destroys Parasitic InfectionsDocument2 pagesPEMF Machine Destroys Parasitic InfectionskjprnewsNo ratings yet
- Definitive Guide To Red Light Therapy PhotobiomodulationDocument10 pagesDefinitive Guide To Red Light Therapy PhotobiomodulationDavid Jenkins0% (2)
- Too Good To Be Truth PDFDocument84 pagesToo Good To Be Truth PDFivica_putNo ratings yet
- 2.about - Humic Fulvic - Substances.1Document35 pages2.about - Humic Fulvic - Substances.1BrianNo ratings yet
- Cause of DiseaseDocument7 pagesCause of Diseaseparacelsus5No ratings yet
- B1 ThiamineDocument8 pagesB1 ThiamineK AnjaliNo ratings yet
- DAT Bootcamp Biology Notes PDFDocument123 pagesDAT Bootcamp Biology Notes PDFgefNo ratings yet
- What Is Fulvic AcidDocument17 pagesWhat Is Fulvic AcidRaul A. ArcangelNo ratings yet
- Trans Dermal MagnesiumDocument2 pagesTrans Dermal Magnesiumkpraj01100% (1)
- Iodine Summaryupdate 2016Document26 pagesIodine Summaryupdate 2016FrankNo ratings yet
- AK Forbidden - Health Protocol K enDocument3 pagesAK Forbidden - Health Protocol K enKhiemNo ratings yet
- MethyleneBlue AnalysisDocument4 pagesMethyleneBlue AnalysisManuelilloNo ratings yet
- Oxidative Stress and AntioxidantsDocument34 pagesOxidative Stress and AntioxidantsTauseefNo ratings yet
- The Purification Of: Dimethylsulphoxide ForDocument7 pagesThe Purification Of: Dimethylsulphoxide ForHasan KoyuncuNo ratings yet
- Protection From Vaxx Shedding - Top Medical Experts Share Proven ProtocolsDocument19 pagesProtection From Vaxx Shedding - Top Medical Experts Share Proven ProtocolsShankar RNo ratings yet
- One Mineral Can Help A Myriad of Conditions From Atherosclerosis To "Copd"To ZitsDocument6 pagesOne Mineral Can Help A Myriad of Conditions From Atherosclerosis To "Copd"To ZitsvanillasmoothieNo ratings yet
- Nothing Boring About BoronDocument14 pagesNothing Boring About BoronBobbyNo ratings yet
- Make Colloidal GoldDocument1 pageMake Colloidal GoldKali100% (1)
- Dmso PDFDocument16 pagesDmso PDFLong Jun100% (1)
- Natural Treatments For Alzheimers 35ppDocument35 pagesNatural Treatments For Alzheimers 35ppaman_arora100% (1)
- Modified Citrus PeptidesDocument34 pagesModified Citrus PeptidesNyala100% (2)
- Ozone TherapyDocument24 pagesOzone TherapyDadoBabylobas100% (2)
- MRSA Eradication Using Chlorine DioxideDocument6 pagesMRSA Eradication Using Chlorine DioxideDr George GeorgiouNo ratings yet
- Shilajit The Panacea For CancerDocument48 pagesShilajit The Panacea For Cancerliving63100% (1)
- Roundup (Glyphosate) is Making You Sicker than You Think: Restore Health - Control Allergies, Autism, Heart and more!From EverandRoundup (Glyphosate) is Making You Sicker than You Think: Restore Health - Control Allergies, Autism, Heart and more!No ratings yet
- Interference Fields: in Healing Chronic IllnessDocument7 pagesInterference Fields: in Healing Chronic IllnessPoorni ShivaramNo ratings yet
- ENZYMES Problems and SolutionsDocument6 pagesENZYMES Problems and SolutionsChadby GraNaNo50% (2)
- Iodine-Weapon Against VirusesDocument5 pagesIodine-Weapon Against ViruseselgrankanNo ratings yet
- Enzyme InhibitionDocument7 pagesEnzyme InhibitionJane Docdoc100% (1)
- Disease Reprieve: Living into the Golden YearsFrom EverandDisease Reprieve: Living into the Golden YearsRating: 5 out of 5 stars5/5 (1)
- Peptide Immunotherapy 1Document14 pagesPeptide Immunotherapy 1cosmicnunNo ratings yet
- How To Reverse Hearing Loss and TinnitusDocument24 pagesHow To Reverse Hearing Loss and TinnitusMark Sloan100% (4)
- Detox - Louisa Williams HandoutDocument3 pagesDetox - Louisa Williams HandoutDaniel SolisNo ratings yet
- Topical Corticosteroid AbuseDocument34 pagesTopical Corticosteroid AbuseDr Daulat Ram DhakedNo ratings yet
- Dmso TinnitusDocument7 pagesDmso TinnitusJoão FrancoNo ratings yet
- Effects of Humic Acid On Animals and HumansDocument12 pagesEffects of Humic Acid On Animals and HumansKeith Nicholson100% (2)
- Fungal Mycotoxin ConnectionDocument2 pagesFungal Mycotoxin Connectionapi-3695725100% (1)
- A Textbook on EDTA Chelation Therapy: Second EditionFrom EverandA Textbook on EDTA Chelation Therapy: Second EditionRating: 4.5 out of 5 stars4.5/5 (2)
- Nitric OxideDocument11 pagesNitric OxidePhan Thanh DoNo ratings yet
- What Is The Difference Between Glutathione and L - GlutathioneDocument2 pagesWhat Is The Difference Between Glutathione and L - GlutathioneAmitav Mishra0% (2)
- Review of sIgADocument4 pagesReview of sIgALuis Castro Xtrm100% (1)
- Farm Bio-Security With Nano Silver Hydrogen Peroxide Based Alstasan SilvoxDocument2 pagesFarm Bio-Security With Nano Silver Hydrogen Peroxide Based Alstasan SilvoxSilver Hydrogen PeroxideNo ratings yet
- BookmarksDocument8 pagesBookmarksRonNo ratings yet
- Methylene BlueDocument3 pagesMethylene BlueApple CruzNo ratings yet
- ATP Blog - Mineral Logic Discovery of - Tracite Fulvic AcidDocument40 pagesATP Blog - Mineral Logic Discovery of - Tracite Fulvic AcidatpfacebookNo ratings yet
- Biomimetic ChemistryDocument29 pagesBiomimetic ChemistryPrabhatNo ratings yet
- CatalysisDocument50 pagesCatalysisnagendra_rdNo ratings yet
- 1st Year Biochemistry Guidance BookletDocument67 pages1st Year Biochemistry Guidance Bookletusama HafeezNo ratings yet
- Data Analysis Integra 700Document48 pagesData Analysis Integra 700Kader SmailiNo ratings yet
- Gate 2010 PDFDocument8 pagesGate 2010 PDFKedar SharmaNo ratings yet
- PG SyllabusDocument328 pagesPG SyllabusHishar Mirsam100% (1)
- Cell Kinetics and Fermenter DesignDocument116 pagesCell Kinetics and Fermenter DesignCriselda Oliva CarinoNo ratings yet
- Diffusion and Biological Reaction in Immobilized Biocatalyst SystemsDocument16 pagesDiffusion and Biological Reaction in Immobilized Biocatalyst SystemsHana HamidNo ratings yet
- Biochemistry: Pharmaglimps-A Glimpse of PharmacyDocument38 pagesBiochemistry: Pharmaglimps-A Glimpse of PharmacySHRIKANTNo ratings yet
- GROUP 7 Che 4101Document64 pagesGROUP 7 Che 4101nica roseNo ratings yet
- Mechaelis-Menten KineticsDocument7 pagesMechaelis-Menten KineticsfayeNo ratings yet
- Properties of Enzyme Inhibition (CH 3, 7)Document18 pagesProperties of Enzyme Inhibition (CH 3, 7)afaf100% (1)
- AsaDocument35 pagesAsamarz95No ratings yet
- Angew Chem Int Ed - 2005 - Blackmond - Reaction Progress Kinetic Analysis A Powerful Methodology For Mechanistic StudiesDocument19 pagesAngew Chem Int Ed - 2005 - Blackmond - Reaction Progress Kinetic Analysis A Powerful Methodology For Mechanistic StudiesFilipi ChalitaNo ratings yet
- Chapter 4 B Inhibition of Enzyme Activity 3rd Year Chemistry Dep'tDocument21 pagesChapter 4 B Inhibition of Enzyme Activity 3rd Year Chemistry Dep'telsaNo ratings yet
- Pegda EnzymeDocument9 pagesPegda EnzymecarolasbdNo ratings yet
- JIPBSV5I307Document5 pagesJIPBSV5I307Yoakim MoraNo ratings yet
- MSC Orgchem Syllabus PVSL 2017 18 For Website PDFDocument41 pagesMSC Orgchem Syllabus PVSL 2017 18 For Website PDFLucky SiddiqueNo ratings yet
- Bioscience Jamia Malia IslamiaDocument20 pagesBioscience Jamia Malia IslamiaRahul MohantyNo ratings yet
- Module 2Document40 pagesModule 2VINCE VITRIOLONo ratings yet
- Solution Brown Solution: Sample Used Time Oxidized Apple Banana Potato GuavaDocument7 pagesSolution Brown Solution: Sample Used Time Oxidized Apple Banana Potato GuavaLaelannie MagpayoNo ratings yet
- Kinetics of Immobilized Enzyme Reactors - Packed Bed and Fluidized Bed.Document8 pagesKinetics of Immobilized Enzyme Reactors - Packed Bed and Fluidized Bed.Thirunavukkarasu ArunachalamNo ratings yet
- Topic 12 Enzyme Kinetics - DKBPDocument22 pagesTopic 12 Enzyme Kinetics - DKBPMaryam ZakiyyahNo ratings yet
- Determination of Acetylcholinesterase ActivityDocument10 pagesDetermination of Acetylcholinesterase ActivityAlex's SustaitaNo ratings yet
- Ebook Catalytic Kinetics Chemistry and Engineering PDF Full Chapter PDFDocument67 pagesEbook Catalytic Kinetics Chemistry and Engineering PDF Full Chapter PDFserafina.rios491100% (23)
- Good Introduction PDFDocument17 pagesGood Introduction PDFMaria AnghelacheNo ratings yet