Professional Documents
Culture Documents
PCR
Uploaded by
Jose F. Ramirez MendozaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PCR
Uploaded by
Jose F. Ramirez MendozaCopyright:
Available Formats
GeneXpert PC
08/24/16 08:50:05
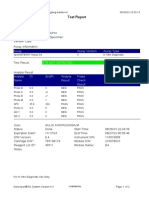
Test Report
Patient ID:
Sample ID:
Test Type:
Sample Type:
C-1559-10 PONCE C.
Specimen
Assay Information
Assay
Assay Version
Assay Type
Xpert MTB-RIF
In Vitro Diagnostic
Test Result:
MTB DETECTED LOW;
Rif Resistance DETECTED
Test and Analyte Result
Ct
EndPt
Analyte
Name
Analyte
Result
Probe
Check
Result
Probe D
26.3
110.0
POS
PASS
Probe C
25.3
145.0
POS
PASS
Probe E
0.0
0.0
NEG
PASS
Probe B
25.6
127.0
POS
PASS
SPC
25.2
311.0
NA
PASS
Probe A
24.7
116.0
POS
PASS
User:
Status:
Expiration Date*:
S/W Version:
Cartridge S/N*:
Reagent Lot ID*:
Notes:
<None>
Done
Start Time:
02/27/11
End Time:
2.1
Instrument S/N:
26893066
Module S/N:
01101
Module Name:
PONCE CONTRERAS FCO.
3302 84 3509
LAVADO BRONQUIAL
UCI-5
10/29/10 07:16:06
10/29/10 08:44:33
706364
609721
A2
For In Vitro Diagnostics Use Only.
GeneXpert Dx System Version 4.4a
Page 1
of 6
GeneXpert PC
08/24/16 08:50:05
Test Report
Error Status:
-
OK
Errors
<None>
Tech. Initial/Date
Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostics Use Only.
GeneXpert Dx System Version 4.4a
Page 2
of 6
GeneXpert PC
08/24/16 08:50:05
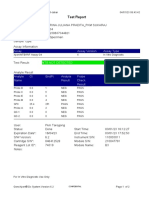
Test Report
Patient ID:
Sample ID:
Test Type:
Sample Type:
RODRIGUEZ SOLIS
Specimen
Assay Information
Assay
Assay Version
Assay Type
Xpert MTB-RIF
In Vitro Diagnostic
Test Result:
MTB DETECTED LOW;
Rif Resistance DETECTED
Test and Analyte Result
Ct
EndPt
Analyte
Name
Analyte
Result
Probe
Check
Result
Probe D
0.0
3.0
NEG
PASS
Probe C
27.8
178.0
POS
PASS
Probe E
29.3
128.0
POS
PASS
Probe B
28.7
141.0
POS
PASS
SPC
27.2
337.0
NA
PASS
Probe A
27.5
142.0
POS
PASS
User:
Status:
Expiration Date*:
S/W Version:
Cartridge S/N*:
Reagent Lot ID*:
Notes:
Error Status:
-
<None>
Done
02/27/11
2.1
26894532
01101
LP
OK
Start Time:
End Time:
Instrument S/N:
Module S/N:
Module Name:
10/20/10 07:57:39
10/20/10 09:26:08
706364
609693
A1
Errors
<None>
For In Vitro Diagnostics Use Only.
GeneXpert Dx System Version 4.4a
Page 3
of 6
GeneXpert PC
08/24/16 08:50:05
Test Report
Tech. Initial/Date
Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostics Use Only.
GeneXpert Dx System Version 4.4a
Page 4
of 6
GeneXpert PC
08/24/16 08:50:05
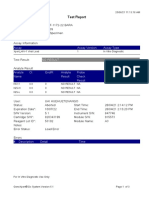
Test Report
Patient ID:
Sample ID:
Test Type:
Sample Type:
Huerta Mejia
Specimen
Assay Information
Assay
Assay Version
Assay Type
Xpert MTB-RIF
In Vitro Diagnostic
Test Result:
MTB DETECTED LOW;
Rif Resistance NOT DETECTED
Test and Analyte Result
Ct
EndPt
Analyte
Name
Analyte
Result
Probe
Check
Result
Probe D
24.1
153.0
POS
PASS
Probe C
23.1
217.0
POS
PASS
Probe E
24.5
130.0
POS
PASS
Probe B
23.6
192.0
POS
PASS
SPC
36.5
133.0
NA
PASS
Probe A
22.6
161.0
POS
PASS
User:
Status:
Expiration Date*:
S/W Version:
Cartridge S/N*:
Reagent Lot ID*:
Notes:
Error Status:
-
<None>
Done
02/27/11
2.1
26894533
01101
Start Time:
End Time:
Instrument S/N:
Module S/N:
Module Name:
10/20/10 07:56:01
10/20/10 09:24:33
706364
609721
A2
OK
Errors
<None>
For In Vitro Diagnostics Use Only.
GeneXpert Dx System Version 4.4a
Page 5
of 6
GeneXpert PC
08/24/16 08:50:05
Test Report
Tech. Initial/Date
Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostics Use Only.
GeneXpert Dx System Version 4.4a
Page 6
of 6
You might also like
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- 31-03-2020 C5 HRSG - LP Reedwater To HRSG DRUM Level Control Valve - PTDocument3 pages31-03-2020 C5 HRSG - LP Reedwater To HRSG DRUM Level Control Valve - PTDave CheungNo ratings yet
- Astec Valves Inspection Certificate SummaryDocument4 pagesAstec Valves Inspection Certificate SummaryShankar RajNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet
- 003 ITP UG PipingDocument4 pages003 ITP UG Pipingrvsingh70100% (1)
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Micom S1 Studio: Settings File Report File: Final - Set Model Number: P543214C3M0570K Printed On: 01/07/2012 12:16:10Document18 pagesMicom S1 Studio: Settings File Report File: Final - Set Model Number: P543214C3M0570K Printed On: 01/07/2012 12:16:10irfuNo ratings yet
- PQR 014 (Qualified Sa 106 GR BDocument5 pagesPQR 014 (Qualified Sa 106 GR Bersenthil100% (1)
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- Site Inspection and Test Record: 13.8/4.16KV UAT-14 TRAFO RTCC PANELDocument6 pagesSite Inspection and Test Record: 13.8/4.16KV UAT-14 TRAFO RTCC PANELAshraf MohammedNo ratings yet
- CT test report analysisDocument5 pagesCT test report analysisSukryadhi SyamriNo ratings yet
- P546 Settings File ReportDocument11 pagesP546 Settings File ReportarunmozhiNo ratings yet
- 21.02.15 Tuv Visit ReportDocument5 pages21.02.15 Tuv Visit ReportssmullaNo ratings yet
- TCR Arabia Company LTD: P.O BOX:3422 DAMAM 31471 (KSA)Document2 pagesTCR Arabia Company LTD: P.O BOX:3422 DAMAM 31471 (KSA)Ronel John Rodriguez CustodioNo ratings yet
- Instrument Configuration (Short Form)Document4 pagesInstrument Configuration (Short Form)shwampaNo ratings yet
- Test ReportDocument5 pagesTest ReportGunawan SiregarNo ratings yet
- O/C & E/F Relay Test Report: - M/S.Grasim Industries Limited Ganjam, OdissaDocument9 pagesO/C & E/F Relay Test Report: - M/S.Grasim Industries Limited Ganjam, OdissaDhivagar NamakkalNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- GC System Suitability ReportDocument8 pagesGC System Suitability ReportShashikant DrShashikant BagadeNo ratings yet
- Certificado UL1703 Celda SolarDocument70 pagesCertificado UL1703 Celda SolarIEE SOLARNo ratings yet
- Annexure 4-Set Files & PSLDocument252 pagesAnnexure 4-Set Files & PSLirfuNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- XRD Scan 20-70V 35mA CuDocument43 pagesXRD Scan 20-70V 35mA CuChandra WijayantiNo ratings yet
- Saudi Arabian Airlines - Staff Bookings - ReservationDocument5 pagesSaudi Arabian Airlines - Staff Bookings - Reservationsari202No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- Transformer Differential TestingDocument39 pagesTransformer Differential Testingerbvp22No ratings yet
- PKM BetoambariDocument6 pagesPKM Betoambarinur liylaNo ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- CEMS FAT Precedure 7131506Document32 pagesCEMS FAT Precedure 7131506Eko Wahyu SetiawanNo ratings yet
- Fine Carbide Tools PTE.LTD. Potential Failure Mode and Effects AnalysisDocument2 pagesFine Carbide Tools PTE.LTD. Potential Failure Mode and Effects AnalysisHeidi Dedication Pader NicolasNo ratings yet
- BL SZ1710025 Test Report PW20L PL2 20L (English)Document10 pagesBL SZ1710025 Test Report PW20L PL2 20L (English)Victoria LiendoNo ratings yet
- C.T Test ReportDocument1 pageC.T Test ReportyazensalehNo ratings yet
- 13205-PPE-DS-V-003-1 - DataSheet - CVDocument7 pages13205-PPE-DS-V-003-1 - DataSheet - CVAnggun RushNo ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- Geekbox V1.23Document21 pagesGeekbox V1.23JocaCarlindeNo ratings yet
- UT Procedure For Longitudinal Seam WeldsDocument8 pagesUT Procedure For Longitudinal Seam WeldsShahul Hameed RazikNo ratings yet
- ITP For CW and ACW Piping WorkDocument8 pagesITP For CW and ACW Piping WorkPhong DoNo ratings yet
- 11-03-2020 C1 Gland Steam Super Heater - UTDocument4 pages11-03-2020 C1 Gland Steam Super Heater - UTDave CheungNo ratings yet
- Definition of Testing Scope (Pu) For Non-Destructive Testing of WeldsDocument1 pageDefinition of Testing Scope (Pu) For Non-Destructive Testing of Weldsاحمد الخزرجيNo ratings yet
- CNT230025 C3 HRSG - Air Tank TCV020&PCV068Document6 pagesCNT230025 C3 HRSG - Air Tank TCV020&PCV068Dave CheungNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- JC International Limited: 1a 1b 1c 2 3 4 5a 5b 5c 6 7 8Document1 pageJC International Limited: 1a 1b 1c 2 3 4 5a 5b 5c 6 7 8Michael OkwuwaNo ratings yet
- CNT230045-6 C3 HRSG Blow Down Vessel - ReportDocument5 pagesCNT230045-6 C3 HRSG Blow Down Vessel - ReportDave CheungNo ratings yet
- 31-03-2020 C1 Generator Rotor Hydrogen Seal Ring - UTDocument6 pages31-03-2020 C1 Generator Rotor Hydrogen Seal Ring - UTDave CheungNo ratings yet
- CNT230022-5 C3 HRSG LP Drum - MTreportDocument6 pagesCNT230022-5 C3 HRSG LP Drum - MTreportDave CheungNo ratings yet
- CovidTestResult ST115495 PANG ConradDocument1 pageCovidTestResult ST115495 PANG ConradChaudhry Salman SerdarNo ratings yet
- Site Inspection and Test Record: 33061701001 30631078/00 Qurayyah-2 P/P 380kV Switching StationDocument1 pageSite Inspection and Test Record: 33061701001 30631078/00 Qurayyah-2 P/P 380kV Switching StationSameer SyedNo ratings yet
- List of Contents: Station: NTPC Simhadri BHEL REF NO: PS-DC-186-500-Ntpc Ref No: Sim/1/Ts/1Document12 pagesList of Contents: Station: NTPC Simhadri BHEL REF NO: PS-DC-186-500-Ntpc Ref No: Sim/1/Ts/1Premkumar VasudevanNo ratings yet
- How-to Manual for Pacemaker and ICD Devices: Procedures and ProgrammingFrom EverandHow-to Manual for Pacemaker and ICD Devices: Procedures and ProgrammingNo ratings yet
- Malignant Pleural EffusionsDocument17 pagesMalignant Pleural EffusionsJose F. Ramirez MendozaNo ratings yet
- Good AfternoonDocument1 pageGood AfternoonJose F. Ramirez MendozaNo ratings yet
- Ingles TaliaDocument4 pagesIngles TaliaJose F. Ramirez MendozaNo ratings yet
- SuperlativosDocument7 pagesSuperlativosJose F. Ramirez MendozaNo ratings yet
- 1 PDFDocument6 pages1 PDFJose F. Ramirez MendozaNo ratings yet
- Fisiopatologia Del SAOS ArticulosDocument1 pageFisiopatologia Del SAOS ArticulosJose F. Ramirez MendozaNo ratings yet
- Sterilization and DisinfectionDocument41 pagesSterilization and DisinfectionqiotenseiNo ratings yet
- Memoir of Glicerio Abad - 2005 - v2Document6 pagesMemoir of Glicerio Abad - 2005 - v2Jane JoveNo ratings yet
- Eastern RlyDocument25 pagesEastern Rlyshivam.jhawar95No ratings yet
- Methyldopa Drug DataDocument3 pagesMethyldopa Drug DataLaurel Joshua Reyes DauzNo ratings yet
- Laboratory Notebook: Aquatic Ecology and ResourcesDocument7 pagesLaboratory Notebook: Aquatic Ecology and ResourcesAireen Diamsim MaltoNo ratings yet
- 2018-06-21 Calvert County TimesDocument24 pages2018-06-21 Calvert County TimesSouthern Maryland OnlineNo ratings yet
- Rabor vs CSC - Retirement age extension disputeDocument2 pagesRabor vs CSC - Retirement age extension disputeGladys Bustria OrlinoNo ratings yet
- QAQC Mechanical EngineerDocument6 pagesQAQC Mechanical EngineerMohammed Anwaar ShaikhNo ratings yet
- RPH Finals Quiz BeeDocument29 pagesRPH Finals Quiz BeeJoshua Liann EscalanteNo ratings yet
- Ch-19 Gas Welding, Gas Cutting & Arc WeldingDocument30 pagesCh-19 Gas Welding, Gas Cutting & Arc WeldingJAYANT KUMARNo ratings yet
- FA SSV3013 - Sem 2 2021 - 22Document4 pagesFA SSV3013 - Sem 2 2021 - 22SITI ZUBAIDAH BINTI HALIMNo ratings yet
- Myth and Folklo-WPS OfficeDocument211 pagesMyth and Folklo-WPS OfficeAryan A100% (1)
- Dentwiton Company Profile (En) 20221019-V2.1Document20 pagesDentwiton Company Profile (En) 20221019-V2.1BRAIS FREIRÍA LORENZONo ratings yet
- Artículo Estudio Ramachandran AGI Personas Bigénero PDFDocument6 pagesArtículo Estudio Ramachandran AGI Personas Bigénero PDFMikaNo ratings yet
- The Political Ecology of Food and AgricultureDocument15 pagesThe Political Ecology of Food and AgricultureMitchNo ratings yet
- Aafreen Project Report SIPDocument19 pagesAafreen Project Report SIPshubham moonNo ratings yet
- Handbook of Heterogeneous Catalytic Hydrogenation For Organic Synthesis 2001 2Document747 pagesHandbook of Heterogeneous Catalytic Hydrogenation For Organic Synthesis 2001 2Purna Bhavnari75% (4)
- Raising Indigenous Chickens in UgandaDocument9 pagesRaising Indigenous Chickens in Ugandashemks79% (38)
- LPT22Document3 pagesLPT22Leonardo Vinicio Olarte CarrilloNo ratings yet
- ODI AmE L1 ExtraGrammarWkshtDocument30 pagesODI AmE L1 ExtraGrammarWkshtJuan Carlos FlorezNo ratings yet
- NSR 125 PriručnikDocument9 pagesNSR 125 PriručnikSenail Mehić50% (2)
- CFSP Example QuestionsDocument5 pagesCFSP Example Questionsuserscribd2011No ratings yet
- Pehealth11 q2 Mod2of2 H.O.P.E v2Document26 pagesPehealth11 q2 Mod2of2 H.O.P.E v2Avillz Mar LeeNo ratings yet
- ICAEW Professional Level Business Planning - Taxation Question & Answer Bank March 2016 To March 2020Document382 pagesICAEW Professional Level Business Planning - Taxation Question & Answer Bank March 2016 To March 2020Optimal Management SolutionNo ratings yet
- Uco Bank Final (Simple Charts)Document40 pagesUco Bank Final (Simple Charts)gopal8726No ratings yet
- KeirseyDocument28 pagesKeirseyapi-525703700No ratings yet
- Intensive English 5: Week 7 Online Session 2 Unit 12: Too Much Work - Unit 13: People With ProblemsDocument19 pagesIntensive English 5: Week 7 Online Session 2 Unit 12: Too Much Work - Unit 13: People With ProblemsHoracioCruiseNo ratings yet
- Operation and Maintenance of Power PlantDocument31 pagesOperation and Maintenance of Power PlantAnkur Pathak100% (1)
- DengueDocument46 pagesDengueMuhammad Ayub KhanNo ratings yet
- Installation and Commissining of TransformerDocument27 pagesInstallation and Commissining of TransformereliahudNo ratings yet