Professional Documents
Culture Documents
Chapter 27
Uploaded by
Yheng GaosaiiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 27
Uploaded by
Yheng GaosaiiCopyright:
Available Formats
1 Mistley Jane
CHAPTER 27: BIOSYNTHESIS OF THE Lengthy Metabolic Pathways Form the Nutritionally Essential Amino
NUTRITIONALLY NONESSENTIAL Acids
AMINO ACIDS The existence of nutritional requirements suggests that dependence on
an external supply of a given nutrient can be of greater survival value
BIOMEDICAL IMPORTANCE than the ability to biosynthesize it. Why?
AMINO ACID DEFICIENCY If a specific nutrient is present in the food, an organism that
states can result if nutritionally essential amino acids are can synthesize it will transfer to its progeny genetic
absent from the diet, or are present in inadequate amount information of negative survival value.
KWASHIORKOR The survival value is negative rather than nil because:
results when a child is weaned onto a starchy diet poor in ATP and
protein
MARASMUS
both caloric intake and specific amino acids are deficient
SHORT BOWEL SYNDROME
unable to absorb sufficient quantities of calories and
nutrients suffer from significant nutritional and metabolic
abnormalities
SCURVY
a dietary deficiency of vitamin C, and specific genetic
disorders are associated with an impaired ability of
connective tissue to form hydroxyproline and hydroxylysine
The resulting conformational instability of collagen results in
bleeding gums, swelling joints, poor wound healing, and
ultimately in death.
MENKES SYNDROME
nutrients are required to synthesize unnecessary DNA
characterized by kinky hair and growth retardation
even if specific encoded genes are no longer expressed
results from a dietary deficiency of copper
The number of enzymes required by prokaryotic cells to
an essential cofactor for the enzyme lysyl oxidase that
synthesize the nutritionally essential amino acids is large
functions in formation of the covalent cross-links that
relative to the number of enzymes required to synthesize the
strengthen collagen fibers
nutritionally nonessential amino acids (Table 272).
GENETIC DISORDERS OF COLLAGEN BIOSYNTHESIS
This suggests a survival advantage in retaining the ability
include several forms of osteogenesis imperfecta
to manufacture easy amino acids while losing the ability
characterized by fragile bones
to make difficult amino acids.
EHLERS-DANLOS SYNDROME
The metabolic pathways that form the nutritionally essential
a group of connective tissue disorders that result in mobile
amino acids occur in plants and bacteria, but not in humans.
joints and skin abnormalities due to defects in the genes that
encode enzymes including lysyl hydroxylase.
BIOSYNTHESIS OF THE NUTRITIONALLY NONESSENTIAL
AMINO ACIDS
NUTRITIONALLY ESSENTIAL &
NUTRITIONALLY NONESSENTIAL AMINO ACIDS
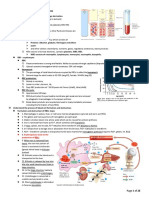
GLUTAMATE
20 Amino Acid Requirements of Humans
common amino acids
Nutritionally Essential Nutritionally the precursor of the so-called glutamate family of amino
are Nonessential essential to ensure
Arginine a
Alanine acids
Histidine Asparagine health.
formed by the reductive amidation of the citric acid cycle -
8 must be present in
Isoleucine Aspartate
Leucine Cysteine
ketoglutarate a reaction catalyzed by:
Lysine Glutamate the human diet, and
Methionine Glutamine mitochondrial glutamate dehydrogenase
thus are best
The reaction strongly favors glutamate synthesis, which
Phenylalanine Glycine
Threonine Hydroxyproline
termed
b
Tryptophan Hydroxylysine
b
lowers the concentration of cytotoxic ammonium ion.
Valine Proline nutritionally
Serine GLUTAMINE
essential.
Tyrosine
The amidation of glutamate to glutamine catalyzed by:
12 amino acids are
glutamine synthetase, involves the intermediate
nutritionally nonessential since they need not be
formation of -glutamyl phosphate
present in the diet.
Following the ordered binding of glutamate and ATP:
The distinction between these two classes of amino acids was
glutamate attacks the -phosphorus of ATP, forming -
established in the 1930s by:
glutamyl phosphate and ADP. NH4+ then binds, and
feeding human subjects purified amino acids in place of
uncharged NH3 attacks -glutamyl phosphate.
protein
Release of Pi and of a proton from the -amino group of
Amino acid deficiency disorders are endemic in certain regions
the tetrahedral intermediate then allows release of the
of West Africa where diets rely heavily on grains that are
product, glutamine.
poor sources of tryptophan and lysine.
ALANINE & ASPARTATE
Nutritional disorders include:
ALANINE: transamination of pyruvate
Kwashiorkor
The amino group may be glutamate or aspartate
Marasmus
The other product thus is, -ketoglutarate or
ARGININE: Nutritionally semiessential. Synthesized at
oxaloacetate
rates inadequate to support growth of children
ASPARTATE: transamination of oxaloacetate
HYDROXYPROLINE&HYDROXYLYSINE: Not necessary for
protein synthesis, but is formed during post-translational
processing of collagen.
BIOCHEMISTRY|CHAPTER 27: BIOSYNTHESIS OF NUTRITIONALLY NONESSENTIAL AMINO ACIDS
2 Mistley Jane
Peptidyl hydroxyproline and hydroxylysine arise from proline
and lysine, but only after these amino acids have been
incorporated into peptides.
Hydroxylation of peptidyl prolyl and peptidyl lysyl residues,
GLUTAMATE DEHYDROGENASE, GLUTAMINE SYNTHETASE & catalyzed by prolyl hydroxylase and lysyl hydroxylase of skin,
AMINOTRANSFERASES PLAY CENTRAL ROLES IN AMINO ACID skeletal muscle, and granulating wounds requires, in addition
BIOSYNTHESIS to the substrate, molecular O2, ascorbate, Fe2+, and -
The combined action of the enzymes glutamate ketoglutarate.
dehydrogenase, glutamine synthetase, and the For every mole of proline or lysine hydroxylated, one mole of
aminotransferase converts: -ketoglutarate is decarboxylated to succinate.
inorganic ammonium ion into the -amino nitrogen of The hydroxylases are mixedfunction oxidases.
amino acids One atom of O2 is incorporated into proline or lysine, the
ASPARAGINE other into succinate.
The conversion of aspartate to asparagine, catalyzed by: A deficiency of the vitamin C required for these two
Asparagine synthetase, resembles the glutamine hydroxylases results in scurvy, in which bleeding gums,
synthetase reaction, but glutamine, rather than swelling joints, and impaired wound healing result from the
ammonium ion, provides the nitrogen. impaired stability of collagen
Bacterial asparagine synthetases can, however, also use VALINE, LEUCINE, & ISOLEUCINE
ammonium ion. While leucine, valine, and isoleucine are all nutritionally
The reaction involves the intermediate formation of aspartyl essential amino acids, tissue aminotransferases reversibly
phosphate interconvert all three amino acids and their corresponding -
The coupled hydrolysis of PPi to Pi by pyrophosphatase, EC keto acids.
3.6.1.1, ensures that the reaction is strongly favored. These -keto acids thus can replace their amino acids in the
SERINE diet.
Oxidation of the -hydroxyl group of the glycolytic SELENOCYSTEINE, THE 21ST AMINO ACID
intermediate 3-phosphoglycerate, catalyzed by: While the occurrence of selenocysteine in proteins is
3-phosphoglycerate dehydrogenase, converts it to 3- uncommon, at least 25 human selenoproteins are known.
phosphohydroxypyruvate Selenocysteine is present at the active site of several human
Transamination and subsequent dephosphorylation then form enzymes that catalyze redox reactions.
serine. Examples include:
GLYCINE thioredoxin reductase,
Glycine aminotransferases can catalyze the synthesis of glutathione peroxidase
glycine from glyoxylate and glutamate or alanine. deiodinase: converts thyroxine to triiodothyronine
Unlike most aminotransferase reactions, these strongly favor Where present, selenocysteine participates in the catalytic
glycine synthesis. mechanism of these enzymes.
Additional important mammalian routes for glycine formation Significantly, the replacement of selenocysteine by cysteine
are from choline and from serine. can actually impair catalytic activity.
PROLINE Impairments in human selenoproteins have been implicated in:
The initial reaction of proline biosynthesis converts the - Tumorigenesis
carboxyl group of glutamate to the mixed acid anhydride of Atherosclerosis
glutamate -phosphat. Keshan disease: selenium deficiency cardiomyopathy
Subsequent reduction forms glutamate -semialdehyde, which Biosynthesis of selenocysteine requires:
following spontaneous cyclization is reduced to l-proline. Cysteine
CYSTEINE selenite (SeO4 2)
not nutritionally essential ATP
cysteine is formed from methionine which is nutritionally a specific tRNA
essential several enzymes
Following conversion of methionine to homocysteine: Serine provides the carbon skeleton of selenocysteine.
homocysteine and serine form: cystathionine SELENOPHOSPHATE:
hydrolysis of cystathionine: forms cysteine and formed from ATP and selenate, serves as the selenium
homoserine donor.
TYROSINE Unlike hydroxyproline or hydroxylysine, selenocysteine arises
Phenylalanine hydroxylase converts phenylalanine to tyrosine. cotranslationally during its incorporation into peptides.
If the diet contains adequate quantities of the nutritionally The UGA anticodon of the unusual tRNA called tRNASec
essential amino acid phenylalanine, tyrosine is nutritionally normally signals STOP.
nonessential. The ability of the protein synthetic apparatus to identify a
However, since the phenylalanine hydroxylase reaction is selenocysteinespecific UGA codon involves the selenocysteine
irreversible: insertion element, a stem-loop structure in the untranslated
dietary tyrosine cannot replace phenylalanine region of the mRNA.
Catalysis by this mixed-function oxidase incorporates one tRNASec is first charged with serine by the ligase that
atom of O2 into the para position of phenylalanine and reduces charges tRNASer.
the other atom to water. Subsequent replacement of the serine oxygen by selenium
Reducing power, provided as tetrahydrobiopterin derives involves selenophosphate formed by selenophosphate
ultimately from NADPH. synthetase.
HYDROXYPROLINE & HYDROXYLYSINE Successive enzyme-catalyzed reactions convert cysteyl-
Hydroxyproline and hydroxylysine occur principally in collagen. tRNASec to aminoacrylyl-tRNASec and then to selenocysteyl-
Since there is no tRNA for either hydroxylated amino acid: tRNASec.
neither dietary hydroxyproline nor dietary hydroxylysine In the presence of a specific elongation factor that
is incorporated during protein synthesis. recognizes
BIOCHEMISTRY|CHAPTER 27: BIOSYNTHESIS OF NUTRITIONALLY NONESSENTIAL AMINO ACIDS
3 Mistley Jane
selenocysteyl-tRNASec, selenocysteine can then be
incorporated into proteins.
BIOCHEMISTRY|CHAPTER 27: BIOSYNTHESIS OF NUTRITIONALLY NONESSENTIAL AMINO ACIDS
You might also like
- Amino Acids & ProteinsDocument12 pagesAmino Acids & ProteinsJearnie Lou Arroyo100% (1)
- Biochem Trans9 LipidsDocument4 pagesBiochem Trans9 LipidsThalia PacamalanNo ratings yet
- Prelims BiochemistryDocument20 pagesPrelims Biochemistrylyn-lynNo ratings yet
- NutritionDocument12 pagesNutritionJamaicaMaeCalagNo ratings yet
- Statutory Declaration of Common-Law Union: Before You Start, Read The Instruction Guide. Type or Print in Black InkDocument1 pageStatutory Declaration of Common-Law Union: Before You Start, Read The Instruction Guide. Type or Print in Black InkyessherazNo ratings yet
- InterneuronsDocument6 pagesInterneuronsRafu DestajoNo ratings yet
- Biochemistry - 2.08 - Gluconeogenesis and Blood Glucose ControlDocument9 pagesBiochemistry - 2.08 - Gluconeogenesis and Blood Glucose ControlJonathan Decena Jr.No ratings yet
- LABLECDocument167 pagesLABLECFrances Riane Simoy100% (1)
- REV Micro HSB RemedsDocument16 pagesREV Micro HSB RemedsPatricia HariramaniNo ratings yet
- St. Luke's College of Medicine - William H. Quasha Memorial: PhysiologyDocument4 pagesSt. Luke's College of Medicine - William H. Quasha Memorial: PhysiologyMavic VillanuevaNo ratings yet
- Guyton Hall PHYSIOLOGY Chapter 17 PDFDocument3 pagesGuyton Hall PHYSIOLOGY Chapter 17 PDFOsman NazirNo ratings yet
- Joint Affidavit of Continuous Cohabitation SchroederDocument1 pageJoint Affidavit of Continuous Cohabitation SchroederJan Rey JabeloraNo ratings yet
- Biochemistry of Cancer Biochemistry of Cancer: DR Madiha Soban BiochemistryDocument36 pagesBiochemistry of Cancer Biochemistry of Cancer: DR Madiha Soban BiochemistryTalha MohsinNo ratings yet
- HistopathDocument7 pagesHistopathDixie DumagpiNo ratings yet
- For All of The Questions Upper LimpDocument22 pagesFor All of The Questions Upper LimpKAAB MEDICS ZONENo ratings yet
- (Hema Manucript) Myelophthisic Anemia FINALCMPDocument6 pages(Hema Manucript) Myelophthisic Anemia FINALCMPJohney Doe100% (1)
- Calcium, Magnesium, and Potassium Homeostasis: OutlineDocument10 pagesCalcium, Magnesium, and Potassium Homeostasis: OutlineMigs MedinaNo ratings yet
- PHARMA 06. Introduction To Autonomic PharmacologyDocument6 pagesPHARMA 06. Introduction To Autonomic PharmacologyCindy Mae MacamayNo ratings yet
- 1.05 Biochemistry Trans - Coenzyme. Cofactors. Prosthetic Grps TRANS v2Document12 pages1.05 Biochemistry Trans - Coenzyme. Cofactors. Prosthetic Grps TRANS v2April AramNo ratings yet
- (HST) Lab Le 3 SamplexDocument13 pages(HST) Lab Le 3 SamplexFrances Dei100% (1)
- Revised Introduction To The Pharmacology of CNS DrugsDocument9 pagesRevised Introduction To The Pharmacology of CNS DrugsJoshua Ty CayetanoNo ratings yet
- Arteries & Circulation (Guyton, Chapter 14-16)Document21 pagesArteries & Circulation (Guyton, Chapter 14-16)Dianne IgnacioNo ratings yet
- Endocrine System - Introduction - Dr. ValerioDocument9 pagesEndocrine System - Introduction - Dr. ValerioMelissa SalayogNo ratings yet
- Biochem 1.5 Bioenergetics PDFDocument7 pagesBiochem 1.5 Bioenergetics PDFlovelots1234No ratings yet
- Apoptosis: Apo: Apart Ptosis: FallenDocument28 pagesApoptosis: Apo: Apart Ptosis: Fallengull naz100% (1)
- Physio Blood TransDocument7 pagesPhysio Blood TransRoderick PalattaoNo ratings yet
- Chapter 16Document9 pagesChapter 16g_komolafeNo ratings yet
- Digestive SystemDocument11 pagesDigestive SystemDanesa MadoNo ratings yet
- 4.03 Intracellular Traffic, Protein Sorting and BiosignalingDocument16 pages4.03 Intracellular Traffic, Protein Sorting and BiosignalingMiguel DomingoNo ratings yet
- (HST) Lab Le 2 SamplexDocument12 pages(HST) Lab Le 2 SamplexFrances Dei100% (1)
- Physio B - General Senses 2Document5 pagesPhysio B - General Senses 2Melissa SalayogNo ratings yet
- Basal Ganglia 1Document7 pagesBasal Ganglia 1Nala MelegritoNo ratings yet
- Intro + Pharmacodynamics 2Document46 pagesIntro + Pharmacodynamics 2Dana E AbuqaudNo ratings yet
- Synthesis of Nanoparticles From Plant ExtractsDocument10 pagesSynthesis of Nanoparticles From Plant ExtractssitinisasyakirinaNo ratings yet
- Physiology LE3 Samplex 2017BDocument8 pagesPhysiology LE3 Samplex 2017BAxel Alvaran100% (1)
- Histology Its Methods of StudyDocument6 pagesHistology Its Methods of StudyA18- Jessa Mae DayagNo ratings yet
- Nsaids, Dmards, Nonopioid Analgesics, Drugs Used in Gout: DR - Minerva P. Calimag, MD - September 6, 2019Document9 pagesNsaids, Dmards, Nonopioid Analgesics, Drugs Used in Gout: DR - Minerva P. Calimag, MD - September 6, 2019Eryll Paolo Alea100% (2)
- Biochemistry Notes For BoardsDocument35 pagesBiochemistry Notes For BoardsUjjwal PyakurelNo ratings yet
- Guyton Hall PHYSIOLOGY Chapter 14 PDFDocument4 pagesGuyton Hall PHYSIOLOGY Chapter 14 PDFOsman NazirNo ratings yet
- 2021 Host Reponse To InfectionDocument38 pages2021 Host Reponse To Infectionmaggie100% (1)
- Guyton Hall PHYSIOLOGY Chapter 18 PDFDocument2 pagesGuyton Hall PHYSIOLOGY Chapter 18 PDFOsman NazirNo ratings yet
- Aubf Lec 2 Trans 2Document4 pagesAubf Lec 2 Trans 2Aj MondejarNo ratings yet
- 2 - Anatomy Ang Histology Buzzwords Oct 2023 Jamaiyah H. Serad - Hadji OsopDocument7 pages2 - Anatomy Ang Histology Buzzwords Oct 2023 Jamaiyah H. Serad - Hadji OsopmikzhiNo ratings yet
- Migs (With Summary) +paoDocument6 pagesMigs (With Summary) +paoMigs MedinaNo ratings yet
- Lymphatic System ReviewerDocument49 pagesLymphatic System ReviewerJess Lejarde100% (1)
- General Senses 1 PDFDocument5 pagesGeneral Senses 1 PDFMelissa SalayogNo ratings yet
- Chapter 24)Document10 pagesChapter 24)Hazel LopezNo ratings yet
- Introduction To CNS PharmacologyDocument49 pagesIntroduction To CNS Pharmacologymatchees-gone rogueNo ratings yet
- Civil Law 1 - Prelim ExamDocument1 pageCivil Law 1 - Prelim ExamClark LimNo ratings yet
- Roche Chemstrip Micral Insert Instruction SheetDocument2 pagesRoche Chemstrip Micral Insert Instruction SheetJaja DavidNo ratings yet
- Trace Elements and Porphyrins - JBDocument7 pagesTrace Elements and Porphyrins - JBVinNo ratings yet
- Inborn Errors of Urea SynthesisDocument14 pagesInborn Errors of Urea SynthesisDaryl Jacob Bigay100% (1)
- APP2 E1 NoteDocument28 pagesAPP2 E1 NotelifecostNo ratings yet
- Pharmacodynamics3 161031154812Document43 pagesPharmacodynamics3 161031154812Adeel Khan100% (1)
- General Reactions Involved in Amino Acid Metabolism: Dr. Dhiraj J TrivediDocument32 pagesGeneral Reactions Involved in Amino Acid Metabolism: Dr. Dhiraj J Trivediendale gebregzabherNo ratings yet
- NSAIDs, DMARDs & Antigout1Document69 pagesNSAIDs, DMARDs & Antigout1Melissa SalayogNo ratings yet
- MED1 Samplex Rationale 9 - Breast ExaminationDocument4 pagesMED1 Samplex Rationale 9 - Breast ExaminationMartina GarciaNo ratings yet
- Peter J Kennelly, Kathleen M Botham, Owen McGuinness, Victor W RodwellDocument6 pagesPeter J Kennelly, Kathleen M Botham, Owen McGuinness, Victor W Rodwellnataliejanep101No ratings yet
- Peter J Kennelly, Kathleen M Botham, Owen McGuinness, Victor W RodwellDocument16 pagesPeter J Kennelly, Kathleen M Botham, Owen McGuinness, Victor W Rodwellnataliejanep101No ratings yet
- HARPERS - VI Biosynthesis of Nutritionally Nonessential Amino AcidsDocument2 pagesHARPERS - VI Biosynthesis of Nutritionally Nonessential Amino AcidsdandiNo ratings yet
- Posterior and Anterior MediastinumDocument6 pagesPosterior and Anterior MediastinumYheng GaosaiiNo ratings yet
- Superior MediastinumDocument4 pagesSuperior MediastinumYheng GaosaiiNo ratings yet
- HeartDocument19 pagesHeartYheng GaosaiiNo ratings yet
- Enzymes: Regulation of Activities Homeostasis CompartmentationDocument1 pageEnzymes: Regulation of Activities Homeostasis CompartmentationYheng GaosaiiNo ratings yet
- Imp Questions 12-Chemistry 2022 (EM) - WingofeducationDocument17 pagesImp Questions 12-Chemistry 2022 (EM) - WingofeducationVickyNo ratings yet
- Elements and CompoundsDocument10 pagesElements and Compoundsvidyasri19No ratings yet
- Flexible and Efficient Hydrometallurgical Recycling of Li-Ion Batteries of Different ChemistryDocument46 pagesFlexible and Efficient Hydrometallurgical Recycling of Li-Ion Batteries of Different Chemistrytaufiq_hidayat_1982No ratings yet
- QAL1 - ABB - AO2000 LS 25 - en - KDocument3 pagesQAL1 - ABB - AO2000 LS 25 - en - KFarhan SattarNo ratings yet
- Economics of ABS Production ProcessesDocument4 pagesEconomics of ABS Production ProcessesfdfNo ratings yet
- PearsonDocument7 pagesPearsonJimmy M TaopanNo ratings yet
- Zinc StearateDocument2 pagesZinc StearateWeda MaharaniNo ratings yet
- 152 809 2 PBDocument12 pages152 809 2 PBsujal jhaNo ratings yet
- Assay of Acetyl Salicylic Acid and Limit of Salicylic Acid.: Title:Quality Assurance of Aspirin Tablets On The Basis ofDocument4 pagesAssay of Acetyl Salicylic Acid and Limit of Salicylic Acid.: Title:Quality Assurance of Aspirin Tablets On The Basis ofSubhash DhungelNo ratings yet
- 5 16 53 605 PDFDocument21 pages5 16 53 605 PDFTanay GuptaNo ratings yet
- Hqfa 513 K 2 Lo 70709560 ECPPTDocument119 pagesHqfa 513 K 2 Lo 70709560 ECPPTHenna KadyanNo ratings yet
- Racemization of Tris Chelate Complexes: Legal NoticeDocument11 pagesRacemization of Tris Chelate Complexes: Legal NoticeDebraj Dhar PurkayasthaNo ratings yet
- Fashion Is Today Incomplete Without DenimDocument5 pagesFashion Is Today Incomplete Without Denimrajivranjan3490No ratings yet
- Valve Chemical - Compatibility - ChartDocument24 pagesValve Chemical - Compatibility - ChartElzeftawyNo ratings yet
- Basic Info About Glaze FormulationsDocument3 pagesBasic Info About Glaze FormulationsThongMaVanNo ratings yet
- Boron Family - BrahmastraDocument38 pagesBoron Family - BrahmastraStevensonNo ratings yet
- Group 15 Elements: - D (Pi) BondDocument7 pagesGroup 15 Elements: - D (Pi) BondSanju PatelNo ratings yet
- FILE NO 3 Exercise 2 Chemical Formula Writing and Naming of Compounds RevDocument2 pagesFILE NO 3 Exercise 2 Chemical Formula Writing and Naming of Compounds RevEJ TaylanNo ratings yet
- AK - Practice Problems Chapter 15 Organic ChemistryDocument10 pagesAK - Practice Problems Chapter 15 Organic ChemistryangelineNo ratings yet
- Acids Bases and Salts IGCSEDocument22 pagesAcids Bases and Salts IGCSEshruti manraiNo ratings yet
- Cbse G-10 Science Lab ManualDocument48 pagesCbse G-10 Science Lab ManualSuhas SadineniNo ratings yet
- Marking Scheme Paper 1 2 3 SBP Trial SPM 2009Document21 pagesMarking Scheme Paper 1 2 3 SBP Trial SPM 2009Mohd Khairul AnuarNo ratings yet
- Bio 024 - Session 5 Sas Nursing (New Format) - WatermarkDocument9 pagesBio 024 - Session 5 Sas Nursing (New Format) - WatermarkMaria Vannesa Anne SalvacionNo ratings yet
- DPHS 2 Year V7 PHS2101 PHS2101, Pharmaceutics-Ii, Unit-IDocument20 pagesDPHS 2 Year V7 PHS2101 PHS2101, Pharmaceutics-Ii, Unit-IRAKISHO WORLDNo ratings yet
- Check For The Presence of Carbohydrates in A Given Analyte.: 4. Solubility TestDocument2 pagesCheck For The Presence of Carbohydrates in A Given Analyte.: 4. Solubility TestYLADE, ERICCA ANDREANo ratings yet
- Gravity of MaterialsDocument1 pageGravity of Materialsck19654840No ratings yet
- Store Stock - Sheet July18Document31 pagesStore Stock - Sheet July18Dharmender YadavNo ratings yet
- Alkaline Earth MetalsDocument8 pagesAlkaline Earth MetalskailashNo ratings yet
- SUMMATIVE ASSESSMENT 2021 - Paper 1Document4 pagesSUMMATIVE ASSESSMENT 2021 - Paper 1brianNo ratings yet
- Exp 1 PDFDocument3 pagesExp 1 PDFwaniNo ratings yet