Professional Documents
Culture Documents
API
Uploaded by
gaunananguyenCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
API
Uploaded by
gaunananguyenCopyright:
Available Formats
API 20E
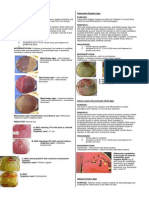
This API-20E test strip (from bioMerieux, Inc.) is used to identify the enteric gram negative
rods (although API makes a variety of other test strips for yeast, Staph, anaerobes, etc.) 20
separate test compartments are on the strip, all dehydrated. A bacterial suspension is used to
rehydrate each of the wells. Some of the wells will have color changes due to pH differences:
others produce end products that have to be identified with reagents. A profile number is
determined from the sequence of + and - test results, then looked up in a codebook having a
correlation between numbers and bacterial species.
OBJECTIVE:
Learn how to perform and interpret the miniaturized, multi-test technique for bacterial
identification.
MATERIALS NEEDED:
agar plates of bacterial species
0.85% NaCl solutions (5ml)
sterile Pasteur pipettes + bulbs
sterile mineral oil
API 20E test strip (for oxidase - gram negative rods)
API test strip incubation chamber AFTER INCUBATION: 10% FeCl3, Barrett's reagents A
and B, Kovac's reagent
PROCEDURE:
Prepare a suspension of the bacteria in the saline tube
1. Inoculate a large colony (2-3mm diameter)of the bacterium (pure culture) into the
0.85% NaCl solution, making sure that the suspension is
homogenous and without clumps of floating bacteria.
2. Use a McFarland barium sulfate standard #3 to quantitate the
suspension and to produce a standard inoculums size..
Inoculate the API strip
1. Holding the strip at a slight angle up from the table top, you will
now inoculate the bacterial suspension into each well with the
sterile pipette.
2. Touch the end of the pipette to the side of the cupule,
allowing capillary action to draw the fluid into the well as
you slowly squeeze the bulb. This should eliminate any
bubbles forming in the wells. Each well should be filled
up to the neck (see diagram).
3. CIT, VP, and GEL have boxes around their names.
These test wells will be filled all the way up to the top of
the well.
4. LDC, ODC, ADH, H2S, and URE are filled as described
in step B, but they will then be filled up to the top with
sterile mineral oil.
Incubate the strip in its chamber
1. The bottom of the incubation chamber has small indented wells in the bottom: fill it with
water just enough to fill these indentations.
2. Place the strip into this bottom. There should not be so much water that it slops onto
the API strip.
3. Place the top of the incubation chamber over the bottom, and label it.
4. Place the strip at 37o C for 18-24 hours.
INTERPRETATION:
1. Add the proper reagents to the
compartments:
o 1 drop of Kovac's to the IND (read within
a couple of minutes)
o 1 drop of Barritt's A and B to VP (a +
reaction may take up to 10 minutes)
o 1 drop of FeCl3 to TDA
2. Read all other tests as described (chart below) without reagents using the table
below..
3. Record results on the diagram handed out to you in lab. The oxidase test reaction
should be negative, and is added as the last test result.
4. Take your result sheet to the computer to INPUT YOUR REACTIONS: The API 20E
website database will provide you with potential organisms.
A more specific 9-digit number can be obtained with a few
more tests:, but NOT DURING THIS LAB.
(pictures from the API 20E documentation, from BioMerieux)
READING THE API 20
TESTS SUBSTRATE REACTION TESTED - RESULTS + RESULTS
ONPG ONPG beta-galactosidase colorless yellow
ADH arginine arginine dihydrolase yellow red/orange
LDC lysine lysine decarboxylase yellow red/orange
ODC ornithine ornithine decarboxylase yellow red/orange
CIT citrate citrate utilization pale green/yellow blue-green/blue
H2S Na thiosulfate H2S production colorless/gray black deposit
URE urea urea hydrolysis yellow red/orange
TDA tryptophan deaminase yellow brown-red
IND tryptophan indole production yellow red (2 min.)
VP Na pyruvate acetoin production colorless pink/red (10 min.)
GEL charcoal gelatin gelatinase no diffusion of black black diffuse
GLU glucose fermentation/oxidation blue/blue-green yellow
MAN mannitol fermentation/oxidation blue/blue-green yellow
INO inositol fermentation/oxidation blue/blue-green yellow
SOR sorbitol fermentation/oxidation blue/blue-green yellow
RHA rhamnose fermentation/oxidation blue/blue-green yellow
SAC sucrose fermentation/oxidation blue/blue-green yellow
MEL melibiose fermentation/oxidation blue/blue-green yellow
AMY amygdalin fermentation/oxidation blue/blue-green yellow
ARA arabinose fermentation/oxidation blue/blue-green yellow
OX oxidase oxidase colorless/yellow violet
QUESTIONS:
1. What is the purpose of the water in the tray?
2. What is the function of the mineral oil?
3. What are the advantages of this test (compared to regular biochemical tube media)?
4. What are the disadvantages of this test?
LAB MANUAL: TABLE OF CONTENTS
8/2009, Jackie Reynolds, Richland College
You might also like
- Api 20eDocument3 pagesApi 20eLuis Ferdinand Dacera-Gabronino Gamponia-NonanNo ratings yet
- Analytical Profile Index: Product Characterisation of TestDocument7 pagesAnalytical Profile Index: Product Characterisation of Testndunghua100% (1)
- Api 20Document25 pagesApi 20خالد الوحيشىNo ratings yet
- Catalase TestDocument12 pagesCatalase Testshiva121294No ratings yet
- Enteropluri Code Book 7-2013Document229 pagesEnteropluri Code Book 7-2013bobNo ratings yet
- Micro 101: Carbohydrate Metabolism LabDocument16 pagesMicro 101: Carbohydrate Metabolism Labmaraki998No ratings yet
- Biochemical TestingDocument10 pagesBiochemical TestingKatrina Mae MedinaNo ratings yet
- Identification of Pseudomonas SPDocument20 pagesIdentification of Pseudomonas SPUttam Kr Patra100% (4)
- SIM TEST IDENTIFIES BACTERIADocument3 pagesSIM TEST IDENTIFIES BACTERIANisha HernandezNo ratings yet
- Determining Enzyme ActivityDocument8 pagesDetermining Enzyme ActivityNesha VincentNo ratings yet
- Merck Rebrand - 105287 - 1906 PDFDocument5 pagesMerck Rebrand - 105287 - 1906 PDFUtama PutraNo ratings yet
- L010570 PDFDocument9 pagesL010570 PDFAnna PianezzolaNo ratings yet
- Enteropluri TestDocument9 pagesEnteropluri TestNerdyPotatoNo ratings yet
- الترشيح الغشائيDocument1 pageالترشيح الغشائيsami algradeeNo ratings yet
- Media Biochemical TestsDocument36 pagesMedia Biochemical TestsIsmail Bazly ZarirNo ratings yet
- KB003Document4 pagesKB003sppNo ratings yet
- Media & Biochemical Tests: Laboratory ObjectivesDocument36 pagesMedia & Biochemical Tests: Laboratory ObjectivesHiba Abu-jumahNo ratings yet
- Microbiolal TestingDocument8 pagesMicrobiolal Testingmalakst200No ratings yet
- Imvic Tests (Indole, Methyl Red, Voges-Proskauer, Citrate) + and H SDocument4 pagesImvic Tests (Indole, Methyl Red, Voges-Proskauer, Citrate) + and H Srandiey john abelleraNo ratings yet
- Lesson 3 Quality Control Sterilization and Disinfection ModuleDocument23 pagesLesson 3 Quality Control Sterilization and Disinfection ModuleTinNo ratings yet
- EnterobacteriaceaeDocument20 pagesEnterobacteriaceaeNarayani MathivananNo ratings yet
- Biochemical TestDocument25 pagesBiochemical TestBiswajit DharuaNo ratings yet
- Lab 8Document28 pagesLab 8Mustafa A. DawoodNo ratings yet
- Biochemical Test of BacteriaDocument33 pagesBiochemical Test of Bacteriaaziskf100% (2)
- Merck Rebrand - 100908 - 1907Document5 pagesMerck Rebrand - 100908 - 1907Paula BautistaNo ratings yet
- Api Rapid 20eDocument3 pagesApi Rapid 20eGUIDO ERNESTO VILLOTA CALVACHINo ratings yet
- Jurnal Kimia OrganikDocument8 pagesJurnal Kimia Organikfeby240200No ratings yet
- Investigatory Project_Comparative Study of the Rate of Fermentation of Various Food MaterialsDocument13 pagesInvestigatory Project_Comparative Study of the Rate of Fermentation of Various Food MaterialsHARSHAL NANDURKARNo ratings yet
- China GB4789.40 2010 Food Microbiological Examination Enterobacter Sakazakii PDFDocument13 pagesChina GB4789.40 2010 Food Microbiological Examination Enterobacter Sakazakii PDFmmoradi55No ratings yet
- The IMViC TestsDocument4 pagesThe IMViC TestsVRampriyaNo ratings yet
- IMVIC TestDocument13 pagesIMVIC TestLaksilu Viduraga Peiris100% (5)
- Biochemical Tests: Laboratory ObjectivesDocument24 pagesBiochemical Tests: Laboratory ObjectivesIsak Isak IsakNo ratings yet
- Merck Rebrand - 110275 - 1906Document5 pagesMerck Rebrand - 110275 - 1906Mochamad Khoirul AnamNo ratings yet
- Identification of Enterobacter aerogenesDocument6 pagesIdentification of Enterobacter aerogeneslagbenektelseNo ratings yet
- Urea Agar Base Differentiates EnterobacteriaDocument2 pagesUrea Agar Base Differentiates EnterobacteriavaibhavNo ratings yet
- IMVICDocument20 pagesIMVICFenny Aulia Sugiana100% (1)
- Microbiology ReviewerDocument8 pagesMicrobiology ReviewerSteph Vee100% (6)
- Batch 2 Ahd Microbio CaseDocument34 pagesBatch 2 Ahd Microbio CaseJAN MICAH A. CATEDRALNo ratings yet
- Asistensi BakterDocument15 pagesAsistensi BakterAwaliatun Nur AzizahNo ratings yet
- Uses of Carbohydrate Fermentation Test: EnterobacteriaceaeDocument12 pagesUses of Carbohydrate Fermentation Test: EnterobacteriaceaeChristyl JoNo ratings yet
- Lab Policies Culture Routine Stool Lab 3105Document5 pagesLab Policies Culture Routine Stool Lab 3105Rajeev PareekNo ratings yet
- Biology Lab Report Organic MoleculesDocument5 pagesBiology Lab Report Organic Moleculesapi-257306447No ratings yet
- Biochemical Tests in EnterobacteriaceaeDocument41 pagesBiochemical Tests in Enterobacteriaceaetummalapalli venkateswara rao100% (2)
- Streptococcus Agalactiae: Biochemical TestingDocument8 pagesStreptococcus Agalactiae: Biochemical TestingAki OtaniNo ratings yet
- Enterobacteriaceae Identification GuideDocument105 pagesEnterobacteriaceae Identification GuideYelsin Denis Fabian AmbrosioNo ratings yet
- Accurately Measure Uric Acid LevelsDocument1 pageAccurately Measure Uric Acid LevelsDharmesh Patel100% (3)
- Enterobacteriaceae: Biochemical ReactionsDocument20 pagesEnterobacteriaceae: Biochemical ReactionslindaprihastiwiNo ratings yet
- Biochem Journal PrintDocument76 pagesBiochem Journal PrintNoor KaimkhaniNo ratings yet
- BitekDocument4 pagesBitekhana4No ratings yet
- R2A Agar (European Pharmacopoeia) Plate Count MediumDocument2 pagesR2A Agar (European Pharmacopoeia) Plate Count MediumRiyon FajarprayogiNo ratings yet
- Amali 2 - Cellular RespirationDocument2 pagesAmali 2 - Cellular RespirationFera Nescafe33% (9)
- Mediu de Cultura Cromogen ColiformiDocument2 pagesMediu de Cultura Cromogen ColiformiGeoemilia1No ratings yet
- Method Analytical ColouringDocument4 pagesMethod Analytical ColouringNOMKHULEKO ALICENo ratings yet
- Biochemical - TestsDocument5 pagesBiochemical - TestsMohsen Haleem100% (1)
- Fecal Coliform TestDocument11 pagesFecal Coliform Test22je1042No ratings yet
- Gel Electrophoresis of ProteinsFrom EverandGel Electrophoresis of ProteinsMichael J DunnNo ratings yet
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- The Fundamentals of Scientific Research: An Introductory Laboratory ManualFrom EverandThe Fundamentals of Scientific Research: An Introductory Laboratory ManualNo ratings yet
- Plant and Animal Bio-Chemistry - Including Information on Amino Acids, Proteins, Pigments and Other Chemical Constituents of Organic MatterFrom EverandPlant and Animal Bio-Chemistry - Including Information on Amino Acids, Proteins, Pigments and Other Chemical Constituents of Organic MatterNo ratings yet
- App FDocument32 pagesApp FgaunananguyenNo ratings yet
- 7 Nguyen Tac ISO 9001Document17 pages7 Nguyen Tac ISO 9001gaunananguyenNo ratings yet
- Quality Manual Table of ContentsDocument7 pagesQuality Manual Table of ContentsFA KhanNo ratings yet
- CoagulaseDocument2 pagesCoagulaseJiki LaluNo ratings yet
- Aplac TR 001 Issue 2Document48 pagesAplac TR 001 Issue 2gaunananguyenNo ratings yet
- Intro TestDocument31 pagesIntro TestsriramNo ratings yet
- Exig 16140 ENDocument36 pagesExig 16140 ENgaunananguyen0% (1)
- Microbiology Principles and Explorations 9Th Edition Black Solutions Manual Full Chapter PDFDocument29 pagesMicrobiology Principles and Explorations 9Th Edition Black Solutions Manual Full Chapter PDFlloydkieran71epfi100% (8)
- Evaluation of Synergistic Effect of Ginger, Garlic, Turmeric Extracts On The Antimicrobial Activity of Drugs Against Bacterial PathogensDocument6 pagesEvaluation of Synergistic Effect of Ginger, Garlic, Turmeric Extracts On The Antimicrobial Activity of Drugs Against Bacterial PathogensYuuRi 'Mira' KazuraNo ratings yet
- VETOMDocument15 pagesVETOMK AnjaliNo ratings yet
- Formulating low-cost culture mediaDocument7 pagesFormulating low-cost culture mediaRNo ratings yet
- Introduction To Microbiology: Min Bahadur KunwarDocument34 pagesIntroduction To Microbiology: Min Bahadur KunwarGanesh PoudelNo ratings yet
- Spawn Production For The Mushroom IndustryDocument20 pagesSpawn Production For The Mushroom IndustryShankar ShankyNo ratings yet
- Basic Culture MediaDocument7 pagesBasic Culture MediaramNo ratings yet
- General Microbiology course syllabus for Mindanao UniversityDocument10 pagesGeneral Microbiology course syllabus for Mindanao UniversityMD Tristan100% (2)
- Microbiology Lab ExercisesDocument71 pagesMicrobiology Lab ExercisesAygul RamankulovaNo ratings yet
- Bsczo 302 PDFDocument232 pagesBsczo 302 PDFNikhil SherawatNo ratings yet
- UPLBDocument1 pageUPLBMatt Jazon T. ReyesNo ratings yet
- 2 Part 1Document99 pages2 Part 1m_luchianNo ratings yet
- aSEPTIC TRANSFERDocument16 pagesaSEPTIC TRANSFERNoor AlqurnaNo ratings yet
- 03 The Evaluation of Antibacterial Activity of Fabrics Impregnated With Dimethyltetradecyl (3 - (Trimethoxysilyl) Propyl) Ammonium ChlorideDocument8 pages03 The Evaluation of Antibacterial Activity of Fabrics Impregnated With Dimethyltetradecyl (3 - (Trimethoxysilyl) Propyl) Ammonium Chloridedharmayanti976No ratings yet
- Waksman Principles of SoilDocument944 pagesWaksman Principles of SoilRadu Iliescu100% (2)
- Handbook For Rhizobia Methods in Legume-RhizobiumDocument511 pagesHandbook For Rhizobia Methods in Legume-RhizobiumAndreina ZamoraNo ratings yet
- Anti Microbial Efficacy For The Esporta Wash SystemDocument15 pagesAnti Microbial Efficacy For The Esporta Wash SystemRobert J. BullockNo ratings yet
- CorneaDocument465 pagesCorneawisinasa100% (2)
- EnumerationDocument7 pagesEnumerationSikin Sikin100% (1)
- Art10 PDFDocument11 pagesArt10 PDFDuong DoanNo ratings yet
- Week 3 Bacterial Smear Preparation and Simple StainDocument2 pagesWeek 3 Bacterial Smear Preparation and Simple StainAngeline LimNo ratings yet
- Infectious CoryzaDocument9 pagesInfectious CoryzaPraveen KumarNo ratings yet
- Isolation of Bacteria From Different Food ProductsDocument51 pagesIsolation of Bacteria From Different Food ProductsAnonymous qyBYAKPNo ratings yet
- LabManual 2009Document324 pagesLabManual 2009brucienzNo ratings yet
- Intervet Inc. D/b/a Merck Animal Health, Et Al., v. Boehringer Ingelheim Vetmedica, Inc., C.A. No. 11-595-LPS (D Del. Dec. 7, 2012)Document17 pagesIntervet Inc. D/b/a Merck Animal Health, Et Al., v. Boehringer Ingelheim Vetmedica, Inc., C.A. No. 11-595-LPS (D Del. Dec. 7, 2012)YCSTBlogNo ratings yet
- Laboratory Exercise 2. Aseptic TechniquesDocument7 pagesLaboratory Exercise 2. Aseptic TechniquesNesly Joy CaballeganNo ratings yet
- Dr.T.V.Rao MDDocument62 pagesDr.T.V.Rao MDDianneMariaNo ratings yet
- Techniques For Studying Bacteria and Fungi PDFDocument32 pagesTechniques For Studying Bacteria and Fungi PDFRoshineeNo ratings yet
- Hygicult Guide To Monitoring CleanlinessDocument36 pagesHygicult Guide To Monitoring Cleanlinessroem1104100% (1)
- Isolation, Screening, and Crude Oil Degradation BacteriaDocument13 pagesIsolation, Screening, and Crude Oil Degradation BacteriakarpanaiNo ratings yet