Professional Documents
Culture Documents
Xercise # 1
Uploaded by
AashiqueOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Xercise # 1
Uploaded by
AashiqueCopyright:
Available Formats
Exercise # 1
Q.1 Ratio of O2 and N2 in a mixture .of these gases is 1 : 4, then what will be the ratio of numbers of molecules in
the mixture of the gases?
(1) 1:4 (2) 7 : 32 (3) 3 : 16 (4) 3:32
Q.2 What should be the ratio of root mean square velocity of molecular hydrogen and molecular oxygen, respec-
tively?
(1) 4 : 1 (2) 1 : 4 (3) 1 : 2 (4) 1 : 8
Q.3 Maximum number of molecules are present in
(1) 54 g of N2O5 (2) 28 g of CO (3) 36 g of H2O (4) 46 g of C2H5OH.
Q.4 Number of atoms in 12 gram of 6C12 is
(1) 6 (2) 6 x 1023 (3) 12 (4) 12 x 6 X 1023
Q.5 A gas at 30 C is Compressed to reduce its volume to half of its initial volume. At what temperature will it
become double of its initial volume?
(1) 60C (2)303C (3) 240C (4) 606C
Q.6 Volume of a gas at 35 C and 1 atmospheric pressure is 3.75 litre. If volume is to be made 3.0 litre at same
pressure, then the temperature will be
(1) 26.6C (2) 0C (3) 3.98C (4) 28C
Q.7 Which of the following is incorrect relation in the critical constants Vc, Tc and Pc?
(1) a = 3 PcVc2 (2) b = Vc/3 (3) Tc = 8a/27Rb (4) b = 3 x Vc
Q.8 At what temperature, the RMS velocities of hydrogen (1 atm. pressure) and oxygen (NTP) will be same
(1) 37 K (2) 17 K (3) 512K (4) 27 K
Q.9 5 grams each of HF, HCI, HBr and HI have been taken at 87 C and 750 mm pressure. Which of the following
should have maximum volume?
(1) HF (2) HCI (3) HBr (4) HI
Q.10 Temperature of 20 lit res of nitrogen at constant pressure is increased from 100 K to 300 K, then the change in
volume will be
(1) 80 litre (2) 60 litre (3) 40 litre (4) 20 litre
Q.11 Value of average free path at 1 atmospheric pressure is L, then what should be its value at 5 atmospheric
pressure?
(1) 2/5 L (2) 5 L (3) L/5 (4) Uncertain
Q.12 Equal molecules of N2 and O2 are kept in a closed container at pressure P. If N2 is removed from the system,
then the pressure will be
(1) P (2) 2P (3) P/2 (4) P2
Q.13 Order of the rate of diffusion of SO2, CO2, PCl3 and SO3 will be
(1) SO2> SO3 > PCl3 > CO2 (2) CO2 > SO2 > SO3 > PCI3
(3) PCl3 > SO3 > SO2 > CO2 (4) CO2 > SO2 > PCl3> SO3
Q.14 What should be the pressure of 2 mole of an ideal gas at 546 K and 44.8 litre volume? .
(1) 2 atm. (2) 1 atm. (3) 4 atm. (4) 5 atm.
Q.15 At 27 C and 750 mm pressure, volume of a gas is 38 ml, then its volume at STP will be
(1) 34 ml (2) 43 ml (3) 4.3 ml (4) 3.4 ml
Q.16 At which temperature, the average velocity of N2 molecules will be equal to the velocity of He molecules at 300
K?
(1) 2100 K (2) 1100 K (3) 420 K (4) None of the above
Q.17 What should be the partial pressure of H2 in a flask, if 2.016 gram of H2 and 16.0 gram of O2 are present in it?
(1) 1/8 of total pressure (2) 1/6 of total pressure
(3) 1/4 of total pressure (4) 2/3 or total pressure
You might also like
- GasousstateDocument10 pagesGasousstatexanshahNo ratings yet
- States of Matter DPPDocument3 pagesStates of Matter DPPs11146366No ratings yet
- Exam 3 Form Code ADocument2 pagesExam 3 Form Code AASinha1No ratings yet
- Neet - Chemistry - States of Matter - 03.07.2023Document5 pagesNeet - Chemistry - States of Matter - 03.07.2023rkshankarNo ratings yet
- State of Matter - 2 - MCQDocument2 pagesState of Matter - 2 - MCQvj jNo ratings yet
- Chemistry (Main) Solution - Code 1Document6 pagesChemistry (Main) Solution - Code 1brijeshNo ratings yet
- Chemistry by SD Sir: TARGET-JEE (Main + Advanced)Document2 pagesChemistry by SD Sir: TARGET-JEE (Main + Advanced)Kripal ChauhanNo ratings yet
- 18c6e3cc33Document2 pages18c6e3cc33Nonis Samuel GerardNo ratings yet
- Part - I: Practice Test-1 (Iit-Jee (Main Pattern) ) : Gaseous StateDocument22 pagesPart - I: Practice Test-1 (Iit-Jee (Main Pattern) ) : Gaseous StatewanderedNo ratings yet
- Chm2045 Final ADocument2 pagesChm2045 Final AChelsea LawrenceNo ratings yet
- Chemistry (Main) Question PaperDocument4 pagesChemistry (Main) Question PaperARVIND MISHRANo ratings yet
- Chem Exam 3 Fall 06Document2 pagesChem Exam 3 Fall 06juliasun8883No ratings yet
- Gases Self Check ProblemsDocument5 pagesGases Self Check ProblemsLissa HannahNo ratings yet
- IIT Jee Mayank Test-2Document5 pagesIIT Jee Mayank Test-2kamalkantmbbsNo ratings yet
- DPP # 1 - 8 Physical ChemistryDocument5 pagesDPP # 1 - 8 Physical ChemistrySankar KumarasamyNo ratings yet
- Ceq Apsp eDocument27 pagesCeq Apsp eChess EnjoyerNo ratings yet
- Gas LawDocument6 pagesGas LawrambabuNo ratings yet
- Sample Questions - Chapter 12Document7 pagesSample Questions - Chapter 12Rasel IslamNo ratings yet
- Multiple Question CHM 101Document26 pagesMultiple Question CHM 101Emmanuella OffiongNo ratings yet
- Test Bank Chapter 5Document7 pagesTest Bank Chapter 5Ahmed ZakiNo ratings yet
- Previous Year Questions (Neet, Aiims, Aipmt, Jipmer)Document3 pagesPrevious Year Questions (Neet, Aiims, Aipmt, Jipmer)abhishekNo ratings yet
- Chemistry: R.S. Stationers, in Association With Connix, BikanerDocument3 pagesChemistry: R.S. Stationers, in Association With Connix, BikanerLakshya ChandakNo ratings yet
- MCQsDocument6 pagesMCQsKashan NoorNo ratings yet
- Mole Concept & Redox ReactionDocument40 pagesMole Concept & Redox ReactionMit ParmarNo ratings yet
- DPP 1 Mole ConceptDocument3 pagesDPP 1 Mole ConceptdhruvNo ratings yet
- LT-23 SPL (G-1) - States of Matter-11-09-21Document8 pagesLT-23 SPL (G-1) - States of Matter-11-09-21orisNo ratings yet
- Gaseos State CPP-3Document5 pagesGaseos State CPP-3Divyansh Jain KingNo ratings yet
- Practice SheetDocument4 pagesPractice SheetJujar YusufNo ratings yet
- CHEM WORKSHEET CH#01 & GASES With Answer KeyDocument7 pagesCHEM WORKSHEET CH#01 & GASES With Answer KeyHabibNo ratings yet
- Class 11 - Chemistry - WPP 2Document5 pagesClass 11 - Chemistry - WPP 225 Mayank SinhaNo ratings yet
- Tutorial 1 - 101117Document1 pageTutorial 1 - 101117Yap Khai Ming OscarNo ratings yet
- LT-23 - SPL - (G-1) - MED-Home Work - States of Matter - 09-09-21Document5 pagesLT-23 - SPL - (G-1) - MED-Home Work - States of Matter - 09-09-21orisNo ratings yet
- YogDocument24 pagesYogYogesh khandelwal0% (1)
- Chang's Test Bank (Chapters 5, 7, 8, 9)Document27 pagesChang's Test Bank (Chapters 5, 7, 8, 9)asfaNo ratings yet
- Neet Weekend Test: ChemistryDocument21 pagesNeet Weekend Test: ChemistryTHARUN THANGELLANo ratings yet
- Eqm TVM SapsDocument6 pagesEqm TVM SapsRaji PradeepNo ratings yet
- Test Bank Chapter 5Document8 pagesTest Bank Chapter 5teafNo ratings yet
- Numerical Questions - Structure of Atom + States of Matter +some Basic Concepts of Chemistry, EquilibriumDocument6 pagesNumerical Questions - Structure of Atom + States of Matter +some Basic Concepts of Chemistry, EquilibriummohammedNo ratings yet
- CHEM 20024 General Chemistry Practice Exam #2Document7 pagesCHEM 20024 General Chemistry Practice Exam #2Yhana Ruth PajitaNo ratings yet
- Neet Test-1 PDFDocument17 pagesNeet Test-1 PDFpremdhimanNo ratings yet
- KCET 2014 Previous Year Paper For ChemistryDocument54 pagesKCET 2014 Previous Year Paper For Chemistrylohith. sNo ratings yet
- Arjuna JEE (2024) : State of MatterDocument3 pagesArjuna JEE (2024) : State of Mattersachidanandkushwaha2468No ratings yet
- Topic Test G10 QP (Quantitative Aspects of Chemical Change 2023) - 1Document7 pagesTopic Test G10 QP (Quantitative Aspects of Chemical Change 2023) - 1ashleymashego88No ratings yet
- Basic ConceptsDocument31 pagesBasic ConceptsZunaira NoreenNo ratings yet
- Middle of Pyramid - Test # 33 - Some Basic Concepts of ChemistryDocument5 pagesMiddle of Pyramid - Test # 33 - Some Basic Concepts of ChemistryJay PatelNo ratings yet
- 061 Chem 101 Final ExamDocument51 pages061 Chem 101 Final ExamIvy GalamitonNo ratings yet
- HW 2 - ChemDocument14 pagesHW 2 - ChemStephanieNo ratings yet
- Gas StateDocument38 pagesGas StatesavisuNo ratings yet
- Mole Concept Udaan DPPDocument16 pagesMole Concept Udaan DPPNareshNo ratings yet
- CEM 141 Final Exam Worksheet AnswersDocument8 pagesCEM 141 Final Exam Worksheet AnswersmotherfuckersyahhhhhhhNo ratings yet
- Test Bank Chapter 5Document9 pagesTest Bank Chapter 5geenah111No ratings yet
- CHEMISTRYCET-16thOCT Ixm5pzgcfy8k2ejcDocument7 pagesCHEMISTRYCET-16thOCT Ixm5pzgcfy8k2ejcanuNo ratings yet
- Chemistry Question Bank - Kinetic Molecular Theory and Gas LawsDocument14 pagesChemistry Question Bank - Kinetic Molecular Theory and Gas Lawsعبدالرحمن شحاتهNo ratings yet
- Class 11 - Chemistry - WPP 1Document5 pagesClass 11 - Chemistry - WPP 125 Mayank SinhaNo ratings yet
- CHEM1070B - Assignment 3 KeyDocument5 pagesCHEM1070B - Assignment 3 Keymakabigail7No ratings yet
- Gas Laws Worksheet IIDocument4 pagesGas Laws Worksheet IIJensen Ryan LimNo ratings yet
- Probset 3LEDocument2 pagesProbset 3LECris Reven GibagaNo ratings yet
- Chapter 13 Study QuestionsDocument2 pagesChapter 13 Study QuestionsКанат ТютеновNo ratings yet
- Chemistry-IIT JEE Mains 2014, 6 April: Topper's Choice Prof. Pawan Babel (PKB)Document6 pagesChemistry-IIT JEE Mains 2014, 6 April: Topper's Choice Prof. Pawan Babel (PKB)Pawan BabelNo ratings yet
- Res and DelocalDocument8 pagesRes and DelocalmadhavNo ratings yet
- Res and DelocalDocument8 pagesRes and DelocalmadhavNo ratings yet
- Res and DelocalDocument8 pagesRes and DelocalmadhavNo ratings yet
- Res and DelocalDocument8 pagesRes and DelocalmadhavNo ratings yet
- Res and DelocalDocument8 pagesRes and DelocalmadhavNo ratings yet
- Res and DelocalDocument8 pagesRes and DelocalmadhavNo ratings yet
- Worksheet - Inverse TrigoDocument6 pagesWorksheet - Inverse TrigoAashiqueNo ratings yet
- Res and DelocalDocument8 pagesRes and DelocalmadhavNo ratings yet
- 1 Centre of MassDocument18 pages1 Centre of MassAashique100% (1)
- Res and DelocalDocument8 pagesRes and DelocalmadhavNo ratings yet
- Applications of Methods of ProofDocument15 pagesApplications of Methods of ProofAashiqueNo ratings yet
- Keep 207Document15 pagesKeep 207Akhilesh Kumar MishraNo ratings yet
- Vsepr TheoryDocument7 pagesVsepr TheoryAashiqueNo ratings yet
- Vsepr TheoryDocument7 pagesVsepr TheoryAashiqueNo ratings yet
- Vsepr TheoryDocument7 pagesVsepr TheoryAashiqueNo ratings yet
- Keep 207Document15 pagesKeep 207Akhilesh Kumar MishraNo ratings yet
- Vsepr TheoryDocument7 pagesVsepr TheoryAashiqueNo ratings yet
- Vsepr TheoryDocument7 pagesVsepr TheoryAashiqueNo ratings yet
- Keep 207Document15 pagesKeep 207Akhilesh Kumar MishraNo ratings yet
- Keep 207Document15 pagesKeep 207Akhilesh Kumar MishraNo ratings yet
- 1Document1 page1AashiqueNo ratings yet
- CL2606 Fluid Mechanics - 1 Introduction and DefinitionsDocument25 pagesCL2606 Fluid Mechanics - 1 Introduction and DefinitionsMarkNo ratings yet
- Compressor HandbookDocument104 pagesCompressor Handbookvincent100% (1)
- Calculations - Guidlines For Control Valve Sizing - SelectionDocument40 pagesCalculations - Guidlines For Control Valve Sizing - SelectionShubham KeniNo ratings yet
- Nombre: Javier Leandro Laura Paxi Codigo: A14423-1 Ci: 8347012Document12 pagesNombre: Javier Leandro Laura Paxi Codigo: A14423-1 Ci: 8347012Leandro JavierNo ratings yet
- ME 231 ch3Document5 pagesME 231 ch3Hassan MrowehNo ratings yet
- CH 1Document29 pagesCH 1Anteneh TarikuNo ratings yet
- Fiscal Metering ExercisesDocument22 pagesFiscal Metering ExercisesCassandra FreyaNo ratings yet
- Fundamentals of Multiphase Flow Chapter 1 - IntroductionDocument4 pagesFundamentals of Multiphase Flow Chapter 1 - IntroductionShivam UpadhyayNo ratings yet
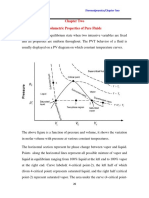
- Chapter Two Volumetric Properties of Pure FluidsDocument18 pagesChapter Two Volumetric Properties of Pure Fluidsson gokuNo ratings yet
- Hagedorn-Brown CorrelationDocument14 pagesHagedorn-Brown CorrelationCaesar DimasDwi SaputriNo ratings yet
- Boyle's Law: ConstantDocument4 pagesBoyle's Law: ConstantChristian CabacunganNo ratings yet
- MP2: Equations of State: Engr. Elisa G. EleazarDocument20 pagesMP2: Equations of State: Engr. Elisa G. EleazarPatrick ValdezNo ratings yet
- hw1 SolutionsDocument3 pageshw1 SolutionsFatih İnalNo ratings yet
- 07pdf 01Document15 pages07pdf 01Satyendra DubeyNo ratings yet
- Gas Flow MeasurementDocument56 pagesGas Flow MeasurementIda Nurdiyana Mohd Yunus100% (1)
- Problem Set 2Document5 pagesProblem Set 2ToMemNo ratings yet
- Em So Quickref ThermDocument2 pagesEm So Quickref ThermAnonymous q4Gyi06No ratings yet
- Nongui, Gelina Anne A. Che 508 - Prelim ExamDocument12 pagesNongui, Gelina Anne A. Che 508 - Prelim ExamDezzerie SanchezNo ratings yet
- Steady or Unsteady Fluid FlowDocument16 pagesSteady or Unsteady Fluid FlowAwadhNo ratings yet
- 1929 - Slater. Physical Meaning of Wave Mechanics (J. Franklin Inst.) 1 PDFDocument7 pages1929 - Slater. Physical Meaning of Wave Mechanics (J. Franklin Inst.) 1 PDFPaulo Victor SouzaNo ratings yet
- Thermodynamics PDFDocument104 pagesThermodynamics PDFasNo ratings yet
- 3 Vacuum Basic ConceptsDocument81 pages3 Vacuum Basic Conceptsapi-3856548100% (3)
- Ideal GasDocument17 pagesIdeal GasPoonamNo ratings yet
- Kelton Calculation DetailsDocument17 pagesKelton Calculation DetailsronziesNo ratings yet
- Heating and Cooling Curve of A SubstanceDocument62 pagesHeating and Cooling Curve of A SubstanceIan Alfred Brimbuela100% (1)
- Greenox Profile IndiaDocument11 pagesGreenox Profile IndiaMuhsen MuhsenNo ratings yet
- Centrifugal Compressor - Wikipedia, The Free EncyclopediaDocument10 pagesCentrifugal Compressor - Wikipedia, The Free EncyclopediaAmbgAmbg100% (1)
- Self-Assessment Exercise 1Document3 pagesSelf-Assessment Exercise 1Deniz YiğitNo ratings yet
- Midterm Exam 1 PDFDocument2 pagesMidterm Exam 1 PDFmilepnNo ratings yet
- Flow and Pressure Drop in Valves and FittingsDocument5 pagesFlow and Pressure Drop in Valves and FittingsĐoàn TrangNo ratings yet