Professional Documents
Culture Documents
Atomic Bonding
Uploaded by
Momo ItachiCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Atomic Bonding
Uploaded by
Momo ItachiCopyright:
Available Formats
Atomic Bonding
(Metallic, Ionic, Covalent, and van
der Waals Bonds)
From elementary chemistry it is known that
the atomic structure of any element is made
up of a positively charged nucleus
surrounded by electrons revolving around it.
An elements atomic number indicates the
number of positively charged protons in the
nucleus. The atomic weight of an atom
indicates how many protons and neutrons in
the nucleus. To determine the number of
neutrons in an atom, the atomic number is
simply subtracted from the atomic weight.
Atoms like to have a balanced electrical charge. Therefore, they usually
have negatively charged electrons surrounding the nucleus in numbers
equal to the number of protons. It is also known that electrons are present
with different energies and it is convenient to consider these electrons
surrounding the nucleus in energy shells. For example, magnesium, with
an atomic number of 12, has two electrons in the inner shell, eight in the
second shell and two in the outer shell.
All chemical bonds involve electrons. Atoms will stay close together if they
have a shared interest in one or more electrons. Atoms are at their most
stable when they have no partially-filled electron shells. If an atom has only
a few electrons in a shell, it will tend to lose them to empty the shell. These
elements are metals. When metal atoms bond, a metallic bond occurs.
When an atom has a nearly full electron shell, it will try to find electrons
from another atom so that it can fill its outer shell. These elements are
usually described as nonmetals. The bond between two nonmetal atoms is
usually a covalent bond. Where metal and nonmetal atom come together
an ionic bond occurs. There are also other, less common, types of bond but
the details are beyond the scope of this material. On the next few pages,
the Metallic, Covalent and Ionic bonds will be covered in more detail.
You might also like
- HAZOP Training290620Document93 pagesHAZOP Training290620NasrulNo ratings yet
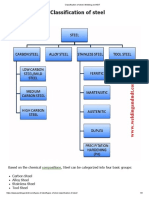
- Classification of Steel - Welding and NDTDocument3 pagesClassification of Steel - Welding and NDTAshif Iqubal100% (1)
- DOSF4001-Body Cream Cleanser Course NotesDocument11 pagesDOSF4001-Body Cream Cleanser Course NotesSiglo Cero Riviera Maya Fer in TulumNo ratings yet
- Chemical Bonding and Molecular StructureDocument13 pagesChemical Bonding and Molecular StructureVishal MalikNo ratings yet
- Crystallography and Mineralogy NotesDocument220 pagesCrystallography and Mineralogy NotesSyed Aquib ShamshadNo ratings yet
- Astm b571 97 R 13pdfDocument4 pagesAstm b571 97 R 13pdfkishor150688No ratings yet
- The Technically Impossible HolocaustDocument20 pagesThe Technically Impossible HolocaustHuckelberry100% (2)
- I11c Instrument Close Loop Check PG 1Document1 pageI11c Instrument Close Loop Check PG 1Momo ItachiNo ratings yet
- Cell Membrane and Cell Transport WebquestDocument6 pagesCell Membrane and Cell Transport WebquestTracy NewKirkNo ratings yet
- Grade 8 Atomic Structure - Notes...Document7 pagesGrade 8 Atomic Structure - Notes...Antonita100% (1)
- Basic Concepts in Electronics ServicingDocument13 pagesBasic Concepts in Electronics ServicingBenjamin FalletNo ratings yet
- Sika Solutions For Concrete BridgesDocument17 pagesSika Solutions For Concrete BridgescaapromoNo ratings yet
- STR A STR PaintingDocument1 pageSTR A STR PaintingMomo ItachiNo ratings yet
- Amb Jul Aug 2015Document100 pagesAmb Jul Aug 2015unitymineNo ratings yet
- AtomDocument4 pagesAtomanjaliNo ratings yet
- Cryocap Air Liquide - BrochureDocument20 pagesCryocap Air Liquide - BrochurePriyanshi VNo ratings yet
- Inspection Test Record (Itr) - A Jacket Pre-Loadout ST08-A Inspection / TestDocument1 pageInspection Test Record (Itr) - A Jacket Pre-Loadout ST08-A Inspection / TestMomo ItachiNo ratings yet
- Atomic Bonding (Metallic, Ionic, Covalent, and Van Der Waals Bonds)Document5 pagesAtomic Bonding (Metallic, Ionic, Covalent, and Van Der Waals Bonds)Aris YusepNo ratings yet
- Coal To LiquidsDocument44 pagesCoal To LiquidsSukaran Singh100% (1)
- Inspection Test Record jacket pre-loadoutDocument1 pageInspection Test Record jacket pre-loadoutMomo ItachiNo ratings yet
- CMTDocument284 pagesCMTAaron R. AllenNo ratings yet
- Atomic Structure and Models ExplainedDocument9 pagesAtomic Structure and Models ExplainedPoojal BatraNo ratings yet
- Anatomy of an atom and basic chemistryDocument5 pagesAnatomy of an atom and basic chemistryKat OrcinoNo ratings yet
- chapter 1 Ele IDocument11 pageschapter 1 Ele Ibiruk satnawNo ratings yet
- Atomic Structure and Interatomic BondingDocument40 pagesAtomic Structure and Interatomic BondingJhomel EberoNo ratings yet
- Element Atom: Ion CharacteristicsDocument3 pagesElement Atom: Ion CharacteristicsClarice Jenn MaltoNo ratings yet
- Chapter Two: Atomic Structure and BondingDocument27 pagesChapter Two: Atomic Structure and Bondingdawit gashuNo ratings yet
- Chemistry Revision Notes-LibreDocument17 pagesChemistry Revision Notes-LibreShridhar MathadNo ratings yet
- Chemical Bonding Lec 3 MSRDocument25 pagesChemical Bonding Lec 3 MSRsiam137032No ratings yet
- Atomic Structure and Chemical Bonding ExplainedDocument9 pagesAtomic Structure and Chemical Bonding ExplainedSoumya Ranjan SahooNo ratings yet
- Chem revision essentialsDocument10 pagesChem revision essentialsmaddieNo ratings yet
- Da-Voc-Abe 3.1.1Document25 pagesDa-Voc-Abe 3.1.1godfrey rufusNo ratings yet
- AtomDocument12 pagesAtomatgimale.comNo ratings yet
- Presentation of Bonding in SolidsDocument14 pagesPresentation of Bonding in SolidsRohit BiswasNo ratings yet
- Introduction To Chemical BondingDocument20 pagesIntroduction To Chemical BondingDe AktivedNo ratings yet
- Definition and Composition of An Atom.Document3 pagesDefinition and Composition of An Atom.Habibu AbdullahiNo ratings yet
- The Structure of Solids: RK ChowdaryDocument27 pagesThe Structure of Solids: RK Chowdaryrkchowdary007No ratings yet
- Chemistry Notes For Form 2Document70 pagesChemistry Notes For Form 2Charles OtienoNo ratings yet
- Atomic Structure EssayDocument4 pagesAtomic Structure EssayholliebaldwinnNo ratings yet
- S9Q2T2L2 Types of Chemical BondingDocument37 pagesS9Q2T2L2 Types of Chemical BondingMark Kevin Cagande EscletoNo ratings yet
- PeriodictableofelementsDocument6 pagesPeriodictableofelementswoodysseusNo ratings yet
- Read and Annotate! I Want To See Notes and Highlights and Underlines On This Paper!Document7 pagesRead and Annotate! I Want To See Notes and Highlights and Underlines On This Paper!api-344880038No ratings yet
- Css Atom StructureDocument20 pagesCss Atom StructureHaris AzizNo ratings yet
- ATOMIC STRUCTURE: PROTONS, NEUTRONS, ELECTRONSDocument4 pagesATOMIC STRUCTURE: PROTONS, NEUTRONS, ELECTRONSE.JohnNo ratings yet
- Victoria Catholic School: Submitted By: Jessica V. MarceloDocument4 pagesVictoria Catholic School: Submitted By: Jessica V. MarceloEric CastilloNo ratings yet
- Protons: Protons Are The Basis of Atoms. While An Atom CanDocument3 pagesProtons: Protons Are The Basis of Atoms. While An Atom CansaadNo ratings yet
- 05 Particles (2) Molecules and Ions 2009Document5 pages05 Particles (2) Molecules and Ions 2009api-270859210% (1)
- 3 Types of Chemical BondsDocument12 pages3 Types of Chemical BondsSaediRisquéBriskeyNo ratings yet
- Atoms, elements, and subatomic particlesDocument2 pagesAtoms, elements, and subatomic particlesJhastine Mhae De VeraNo ratings yet
- Enlaces InteratómicosDocument40 pagesEnlaces InteratómicosNAYELI VIVEROS CRUZNo ratings yet
- Introduction To Chemical BondingDocument5 pagesIntroduction To Chemical BondingarjunvistaNo ratings yet
- Pptx5 Chemical BondingDocument39 pagesPptx5 Chemical BondingLumbay, Jolly MaeNo ratings yet
- Chemistry of LifeDocument14 pagesChemistry of LifeRebecca TercianoNo ratings yet
- Chemistry of LifeDocument14 pagesChemistry of LifeBayramNo ratings yet
- EnE 250 Air Quality Management and Pollution Control Lecture 03 - 2 Characterizing Air Pollution Oct 2015Document115 pagesEnE 250 Air Quality Management and Pollution Control Lecture 03 - 2 Characterizing Air Pollution Oct 2015Alexis Bryan RiveraNo ratings yet
- Ayena Semiconductor DevicesDocument2 pagesAyena Semiconductor DevicesAceng PikriNo ratings yet
- Mariano Marcos State University: PCHM 121: Pharmaceutical Inorganic Chemistry With Qualitative AnalysisDocument14 pagesMariano Marcos State University: PCHM 121: Pharmaceutical Inorganic Chemistry With Qualitative AnalysisKaizenNo ratings yet
- Basic Concepts: Electricity-Ohm's Law - Law of ResistanceDocument8 pagesBasic Concepts: Electricity-Ohm's Law - Law of ResistanceBSMK60No ratings yet
- Safari - Dec 31, 2023 at 3:47 AM 2Document1 pageSafari - Dec 31, 2023 at 3:47 AM 2syansyncNo ratings yet
- Chapter 1, REVIEW OF QUONTAM THEORYDocument13 pagesChapter 1, REVIEW OF QUONTAM THEORYPAUL NDIRITUNo ratings yet
- YR10 - Exam Notes - ChemistryDocument5 pagesYR10 - Exam Notes - Chemistrysmokey.prism.9482No ratings yet
- CHEM 212 Second LectureDocument2 pagesCHEM 212 Second LectureKazeem Ibrahim BelloNo ratings yet
- ETPaper 2Document159 pagesETPaper 2shid kumarNo ratings yet
- Ece1: Electronic Devices and Circuits: Semiconductor BasicsDocument71 pagesEce1: Electronic Devices and Circuits: Semiconductor BasicsAdriel JohnNo ratings yet
- Topic 3. Additional NotesDocument28 pagesTopic 3. Additional NotesChai MingzeNo ratings yet
- Chapter 4-Student Reading: Parts of The AtomDocument12 pagesChapter 4-Student Reading: Parts of The AtomShimmy LimmyNo ratings yet
- Chapter 2Document19 pagesChapter 2Melanie AquinoNo ratings yet
- Mod 2 Book 1 PhysicsDocument41 pagesMod 2 Book 1 Physicsranjit prasadNo ratings yet
- Atom Is The Smallest Part of An Element. An Atom Consists of A Nucleus and Electrons Rotating Around The NucleusDocument29 pagesAtom Is The Smallest Part of An Element. An Atom Consists of A Nucleus and Electrons Rotating Around The NucleusMohammad ShahedNo ratings yet
- Chemical Bond and Its TypesDocument5 pagesChemical Bond and Its TypesKhan NiaziNo ratings yet
- HyberdizationDocument62 pagesHyberdizationapi-3764139100% (1)
- I13c Process Analyser Test PG 1Document1 pageI13c Process Analyser Test PG 1Momo ItachiNo ratings yet
- I13c Process Analyser Test PG 2 PDFDocument1 pageI13c Process Analyser Test PG 2 PDFMomo ItachiNo ratings yet
- I11c Instrument Close Loop Check PG 4 PDFDocument1 pageI11c Instrument Close Loop Check PG 4 PDFMomo ItachiNo ratings yet
- I16c Fire & Gas Device PG 1Document1 pageI16c Fire & Gas Device PG 1Momo ItachiNo ratings yet
- I11c Instrument Close Loop Check PG 2Document1 pageI11c Instrument Close Loop Check PG 2Momo ItachiNo ratings yet
- STR A STR Preload 4Document1 pageSTR A STR Preload 4Momo ItachiNo ratings yet
- I08c ICP ESD Station Test PG 2 PDFDocument1 pageI08c ICP ESD Station Test PG 2 PDFMomo ItachiNo ratings yet
- I11c Instrument Close Loop Check PG 3Document1 pageI11c Instrument Close Loop Check PG 3Momo ItachiNo ratings yet
- Inspection Test Record (Itr) - A Jacket Pre-Loadout ST08-A Inspection / TestDocument1 pageInspection Test Record (Itr) - A Jacket Pre-Loadout ST08-A Inspection / TestMomo ItachiNo ratings yet
- I08c ICP ESD Station Test PG 1Document1 pageI08c ICP ESD Station Test PG 1Momo ItachiNo ratings yet
- Inspection Test Record (Itr) - A Jacket Pre-Loadout ST08-ADocument1 pageInspection Test Record (Itr) - A Jacket Pre-Loadout ST08-AMomo ItachiNo ratings yet
- Riser Inspection Test Record ST05-ADocument1 pageRiser Inspection Test Record ST05-AMomo ItachiNo ratings yet
- STR A STR Passive FireDocument1 pageSTR A STR Passive FireMomo ItachiNo ratings yet
- ITR-B (Piping) Witness Joint1Document1 pageITR-B (Piping) Witness Joint1Momo ItachiNo ratings yet
- Neutral Earthing Resistor E34-A Inspection Test Record (Itr) - ADocument1 pageNeutral Earthing Resistor E34-A Inspection Test Record (Itr) - AMomo ItachiNo ratings yet
- Inspection Test Record (Itr) - A Structural Jacket ST04-ADocument1 pageInspection Test Record (Itr) - A Structural Jacket ST04-AMomo ItachiNo ratings yet
- Inspection Test Record (Itr) - A Structural Members ST01-ADocument1 pageInspection Test Record (Itr) - A Structural Members ST01-AMomo ItachiNo ratings yet
- Overall Final Documentation Summary TrackDocument1 pageOverall Final Documentation Summary TrackMomo ItachiNo ratings yet
- ITR-B (Mecanical) M35BDocument1 pageITR-B (Mecanical) M35BMomo ItachiNo ratings yet
- Inspection Test Record (Itr) - A I & J Tube ST06-ADocument1 pageInspection Test Record (Itr) - A I & J Tube ST06-AMomo ItachiNo ratings yet
- ITR-A E TrasmissionDocument1 pageITR-A E TrasmissionMomo ItachiNo ratings yet
- ITR-A E Gas Turbine GeneratorDocument1 pageITR-A E Gas Turbine GeneratorMomo ItachiNo ratings yet
- ITR-A E Submarine CableDocument1 pageITR-A E Submarine CableMomo ItachiNo ratings yet
- ITR-B (Mecanical) M44BDocument1 pageITR-B (Mecanical) M44BMomo ItachiNo ratings yet
- Inspection record for jacket post loadoutDocument1 pageInspection record for jacket post loadoutMomo ItachiNo ratings yet
- ITR-C (Instrument) 16cDocument1 pageITR-C (Instrument) 16cMomo ItachiNo ratings yet
- The Radio Chemistry of Mercury - Us AECDocument211 pagesThe Radio Chemistry of Mercury - Us AEClondonbluetopazNo ratings yet
- Matriculation Chemistry (Reaction Kinetics) Part 1Document13 pagesMatriculation Chemistry (Reaction Kinetics) Part 1ridwan100% (2)
- ATCO Gas STOPAQ-Vinylester Procedure Rev0Document45 pagesATCO Gas STOPAQ-Vinylester Procedure Rev0mkash028No ratings yet
- T.9.2.7 Reinforcing For Shear - EUROCODE2: RAPT User ManualDocument16 pagesT.9.2.7 Reinforcing For Shear - EUROCODE2: RAPT User Manualtailieuxaydung2019No ratings yet
- Computer Code For Monte Carlo MarchingDocument78 pagesComputer Code For Monte Carlo MarchingJacob H. (Jack) LashoverNo ratings yet
- IR Inspection Program For Fired Heater Mechanical Integrity: New Product!Document1 pageIR Inspection Program For Fired Heater Mechanical Integrity: New Product!bazil17No ratings yet
- Surface TensionDocument25 pagesSurface TensionIshani Gupta100% (1)
- Charles Law ExplainedDocument3 pagesCharles Law ExplainedKaren May UrlandaNo ratings yet
- Novabrite RGB Full Color High Power Led Application Note: R&D CenterDocument15 pagesNovabrite RGB Full Color High Power Led Application Note: R&D CenterVinu KumarNo ratings yet
- Date Planned: - / - / - Daily Tutorial Sheet-13 Expected Duration: 30 Min Actual Date of Attempt: - / - / - Level-3 Exact DurationDocument1 pageDate Planned: - / - / - Daily Tutorial Sheet-13 Expected Duration: 30 Min Actual Date of Attempt: - / - / - Level-3 Exact DurationShahina NasreenNo ratings yet
- Pharmaceutical ChemistryDocument21 pagesPharmaceutical ChemistryBISMA RAFIQNo ratings yet
- Pesticides: - Are Compounds Used To Control Pest (The Latin Word Cida Means To Cut or Kill) - It Could BeDocument13 pagesPesticides: - Are Compounds Used To Control Pest (The Latin Word Cida Means To Cut or Kill) - It Could BeEzra EzraNo ratings yet
- Kanthal Appliance Heating Alloys Handbook PDFDocument33 pagesKanthal Appliance Heating Alloys Handbook PDFwillwNo ratings yet
- Efficient Synthesis of 3-Hydroxy-1,4-Benzodiazepines Oxazepam and Lorazepam by New Acetoxylation Reaction of 3-Position of 1,4-Benzodiazepine Ring - Organic Process Research & DevelopmentDocument12 pagesEfficient Synthesis of 3-Hydroxy-1,4-Benzodiazepines Oxazepam and Lorazepam by New Acetoxylation Reaction of 3-Position of 1,4-Benzodiazepine Ring - Organic Process Research & DevelopmentSimon GeschwindNo ratings yet
- AVENDAÑO, Jay Russell A. Written Report PURUGGANAN, Stephanie Claire SISON, KellyDocument4 pagesAVENDAÑO, Jay Russell A. Written Report PURUGGANAN, Stephanie Claire SISON, KellyKelly SisonNo ratings yet
- Afa Reviewer ADocument28 pagesAfa Reviewer AJane CastroNo ratings yet
- Optek TOP5 Brewery Applications EnglishDocument16 pagesOptek TOP5 Brewery Applications EnglishoptekNo ratings yet
- Chemical Bonding and Molecular Structure - Lecture NotesDocument51 pagesChemical Bonding and Molecular Structure - Lecture NotesEdith EatonNo ratings yet
- 14 CH242 Conjugated & UVDocument72 pages14 CH242 Conjugated & UVrizqiaNo ratings yet