Professional Documents
Culture Documents
at Gamma GT
Uploaded by
Nia MarianiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
at Gamma GT
Uploaded by
Nia MarianiCopyright:
Available Formats
BIOLABO REAGENTS
www.biolabo.fr
GAMMA-GT
Soluble GPNA
MANUFACTURER:
BIOLABO SA,
Reagent for quantitative determination of
02160, Maizy, France γ-glutamyltransferase activity [ EC 2.3.2.2 ] in human serum and plasma.
REF 80110 R1 8 x 30 mL R2 8 x 30 mL
REF 80210 R1 1 x 105 mL R2 10 x 10 mL
REF 80310 R1 10 x 100 mL R2 10 x 100 mL
|
TECHNICAL SUPPORT AND ORDERS
Tel : (33) 03 23 25 15 50
IVD IN VITRO DIAGNOSTIC USE
Fax : (33) 03 23 256 256
CLINICAL SIGNIFICANCE (1) (2) REAGENT PREPARATION
Even though renal tissue has the highest level of γ-glutamyltransferase Use a non-sharp instrument to remove aluminium cap.
(GGT), the enzyme present in serum appears to originate primarily REF 80210: Add promptly 10 mL of vial R1 (Buffer) into the contents
from the hepatobiliary system. GGT activity is elevated in any and all of vial R2 (Substrate).
forms of liver disease. It is highest in cases of intrahepatic or
posthepatic biliary obstruction. It is more sensitive than ALP and Others REF : Add promptly the contents of vial R2 (Substrate) into vial
transaminases in detecting obstructive jaundice, cholangitis and R1 (Buffer).
cholecystitis. Only moderate elevation occurs in infectious hepatitis, Mix gently and wait for complete dissolution before using reagent
chronic alcoholism or drugs use (sedatives, anticonvulsants,and (approximately 2 minutes).
tranquilisers). Decreased GGT activity are found in case of
hypothyroidism.
In summary, GGT is the most sensitive enzymatic indicator of STABILITY AND STORAGE
hepatobiliary disease available at present but doesn’t allow to Store away from light, well cap in the original vial at 2-8°C
discriminate between diiferent kind of liver disease. • Unopened, reagents are stable until expiry date stated on the label
of the kit when stored and used as described in the insert.
PRINCIPLE (4) (5) • Once reconstituted, working reagent is stable for 30 days when free
Szasz, Rosalki and Tarlow method. Reaction scheme is as follows : from contamination.
• Discard any reagent if cloudy or if reagent blank at 405 nm is >
L-G-Glutamyl-p-nitroanilide GGT 0.400.
L-G-Glutamyl-glycylglycine
+ Glycylglycine + p-nitroaniline • Don’t use working reagent after expiry date stated on the label of the
Kit.
The rate of formation of p-nitroaniline, directly proportional to
GGT activity in the specimen, is measured at 405 nm. SPECIMEN COLLECTION AND HANDLING (1) (2)
Unhemolysed serum or EDTA-plasma (up to 1 mg/ml of blood).
Heparin produces turbidity in the reaction mixture; citrate, oxalate and
REAGENTS COMPOSITION fluoride depress GGT activity by 10 to 15%.
Vial R1 BUFFER GGT is stable in serum for :
• 1 month at 2-8°C
Glycylglycine 62 mmol/L • 1 year at–20°C.
TRIS pH 8.1 95 mmol/L
Preservative
INTERFERENCES (1) (3)
Vial R2 SUBSTRATE Hemolysis interferes with the test
For a more comprehensive review of factors affecting this assay refer to
L-G-glutamyl-p-nitroanilide 2 mmol/L the publication of Young D.S.
(GPNA)
MATERIALS REQUIRED BUT NOT PROVIDED
SAFETY CAUTIONS 1. Medical analysis laboratory equipment.
BIOLABO reagents are designated for professional, in vitro diagnostic 2. Normal and pathological control sera.
use.
• Verify the integrity of the contents before use.
• Nitroaniline derivatives (product of the reaction) are toxic. Do not CALIBRATION
inhalate, do not ingest. Results will depend on the accuracy of the instrument calibration, the
• Use adequate protections (overall, gloves, glasses). time counting, the respect of reagent/specimen ratio and the
• Do not pipette by mouth. temperature control.
• In case of contact with skin or eyes, thoroughly wash affected areas • Use the theoretical calibration factor (§ CALCULATION)
with plenty of water and seek medical advice. • Or REF 95015 BIOLABO Multicalibrator (calibration value
• Reagents contain sodium azide (concentration < 0,1%) which may determined with validated statistical technics and metrologically
react with copper and lead plumbing. Flush with plenty of water controlled instrument)
when disposing. 1. or a multiparametric calibrator traceable to a reference method or
• Material Safety Data Sheet is available upon request. material.
• Waste disposal : Respect legislation in force in the country.
All specimens should be handled as potentially infectious, in
accordance with good laboratory practices using appropriate
precautions. Respect legislation in force in the country.
Made in France Latest revision : www.biolabo.fr Revision : 25/07/2011
QUALITY CONTROL MANUAL PROCEDURE
• BIOLABO EXATROL-N (Level I), REF 95010 Let stand reagents and specimens at room temperature.
• BIOLABO EXATROL-P (Level II), REF 95011
• Other assayed control sera referring to the same method. Pipette into 1 cm pathlength thermostated cuvette :
• External quality control program.
It is recommended to control in the following cases : Reagent 1 mL
• At least once a run. Bring to 37°C (30°C) then add :
• At least once within 24 hours.
• When changing vial of reagent. Specimen or Calibrator 50 µL
• After maintenance operations on the instrument.
If control is out of range, apply following actions : Mix. Read initial absorbance after 30 seconds, record absorbance at 405 nm
1. Repeat the test with the same control. every minutes during 3 minutes.
2. If control is still out of range, prepare a fresh control serum and Calculate absorbance change per minute (∆Abs/min).
repeat the test.
3. If control is still out of range, verify analysis parameters : Notes : Specific procedures are available upon request for
Wavelengh, temperature, specimen/reagent ratio, time counting, automated instruments. Please contact BIOLABO technical
calibration factor. support.
4. If control is still out of range, use a new vial of reagent and
reassay
5. If control is still out of range, please contact BIOLABO technical CALCULATION
support or your local Agent.
Calculate the result as follows :
EXPECTED VALUES (2)
With theoretical factor :
Adult GGT activity, measured at 37°C
∆Abs./min.) x 2121
IU/L = (∆
Men (IU/L) 2-30
µkat/L = IU/L
Women (IU/L) 1-24
60
Each laboratory should establish its own normal ranges for the
population it serves.
With seric multicalibrator
GGT Activity = (∆Abs/min) Assay x Calibrator Concentration
PERFORMANCE CHARACTERISTICS (∆Abs/min) Calibrator
Within-run Run to run
N = 30 Normal level High level N = 37 Normal level High level
REFERENCES
Mean IU/L 29.2 115.1 Mean IU/L 32.7 188.4 (1) TIETZ N.W. Text book of clinical chemistry, 3rd Ed. C.A. Burtis, E.R.
S.D. IU/L 0.405 2.6 S.D.IU/L 0.38 1.95 Ashwood, W.B. Saunders (1999) p. 686-689.
(2) Clinical Guide to Laboratory Test, 4th Ed., N.W. TIETZ (2006) p. 470-473..
C.V. % 1.39 2.25 C.V. % 1.16 1.04 th
(3) YOUNG D.S., Effect of Drugs on Clinical laboratory Tests, 4 Ed. (1995) p.
3-296 à 3-300
Detection limit : approximately 4 IU/L
(4) SZASZ G., Clin. Chem., (1969), 15, p.124
Sensitivity for 21 IU/L : approximately 0.010 ∆Abs/min (5) SZASZ G., Methods of enzymatic analysis, (1974) 2cde éd., 2, p.715
Comparison studies with commercially available reagent :
y = 1.003 x – 4.7246 r = 0.9964
LINEARITY
The assay is linear up to 320 IU/L (5.33 µkat/L).
Above, dilute specimen with saline solution and reassay taking into
account the dilution factor. Linearity will depend on the
specimen/reagent ratio.
IVD REF LOT →
Manufacturer Use by In vitro diagnostic Temperature limitation Catalogue number See insert Batch number Store away from light sufficient for dilute with
Made in France Latest revision : www.biolabo.fr Revision : 25/07/2011
You might also like
- GGT Activity TestDocument2 pagesGGT Activity Testمحمد رحيم حسن محمودNo ratings yet
- GAMMA-GT Carboxy GPNA: BiolaboDocument2 pagesGAMMA-GT Carboxy GPNA: BiolaboFariz KasyidiNo ratings yet
- GGT enDocument2 pagesGGT enKaoueche OmarNo ratings yet
- Alkaline Phosphatase Activity Test KitDocument2 pagesAlkaline Phosphatase Activity Test KitRury Darwa Ningrum100% (1)
- GGT InsertDocument2 pagesGGT InsertsharmashyamsinghNo ratings yet
- 1126005I Rev. en KR 04Document2 pages1126005I Rev. en KR 04Nguyễn HuynhNo ratings yet
- Liquick Cor-GGT: Diagnostic Kit For Determination of - Glutamyltransferase ActivityDocument2 pagesLiquick Cor-GGT: Diagnostic Kit For Determination of - Glutamyltransferase ActivityIvana BajunovicNo ratings yet
- Multiparametric Calibrator: Biolabo MulticalibratorDocument1 pageMultiparametric Calibrator: Biolabo MulticalibratorFariz KasyidiNo ratings yet
- Kit InsertDocument2 pagesKit InsertfaujraneNo ratings yet
- Reagen Kit DiasysDocument2 pagesReagen Kit DiasysNiken CahyaningrumNo ratings yet
- Amilase PDFDocument2 pagesAmilase PDFFariz KasyidiNo ratings yet
- Ifu Iga 41 14Document5 pagesIfu Iga 41 14Wesam TayaNo ratings yet
- At 80009Document2 pagesAt 80009Abrian Aziz KNo ratings yet
- Protéines Totales Biuret - ENDocument2 pagesProtéines Totales Biuret - ENYousra NanoNo ratings yet
- Creatinine: Kinetic MethodDocument2 pagesCreatinine: Kinetic MethodCarina AngelNo ratings yet
- EN GGT BAOSR6x19 USDocument2 pagesEN GGT BAOSR6x19 USDharmesh PatelNo ratings yet
- TOTAL PROTEIN Biuret Method: BiolaboDocument2 pagesTOTAL PROTEIN Biuret Method: BiolaboVenura VishwajithNo ratings yet
- GGT 110 - 330 XL-1000 - Xsys0011 - 77 - HDocument4 pagesGGT 110 - 330 XL-1000 - Xsys0011 - 77 - HMatibar RahmanNo ratings yet
- Kit Insert Magnesium (Calmagite)Document2 pagesKit Insert Magnesium (Calmagite)rai citaNo ratings yet
- At 80027Document2 pagesAt 80027Yesha Bio ValenciaNo ratings yet
- Alkaline Phosphatase FS : Order Information SpecimenDocument3 pagesAlkaline Phosphatase FS : Order Information SpecimenmnemonicsNo ratings yet
- ALT - (GPT) Test Adv. Clinical BiochemicalDocument2 pagesALT - (GPT) Test Adv. Clinical BiochemicalAli AlhamdaniNo ratings yet
- Asat (Got) Fs : Order Information Warnings and PrecautionsDocument6 pagesAsat (Got) Fs : Order Information Warnings and PrecautionsTesya PratiwiNo ratings yet
- BioSystems GGTDocument1 pageBioSystems GGTIvana BajunovicNo ratings yet
- ALT GPT (IFCC) Single Vial: BiolaboDocument2 pagesALT GPT (IFCC) Single Vial: Biolabowindy ajengNo ratings yet
- Total Protein FS : Order InformationDocument2 pagesTotal Protein FS : Order InformationMikko DecolongonNo ratings yet
- Ifu BX e Bil Total 1Document6 pagesIfu BX e Bil Total 1Aditya FerdyNo ratings yet
- Pglu-Pap GB-D 21 001Document4 pagesPglu-Pap GB-D 21 001akaweadeNo ratings yet
- AT 22010 HbA1cDocument2 pagesAT 22010 HbA1cMohammed R.HusseinNo ratings yet
- Alat (GPT) Fs : Order Information Warnings and PrecautionsDocument3 pagesAlat (GPT) Fs : Order Information Warnings and Precautionssovi haswindhaNo ratings yet
- Principle of The Method Quality Control: Alkaline PicrateDocument1 pagePrinciple of The Method Quality Control: Alkaline PicrateRisqon Anjahiranda Adiputra100% (1)
- at Bili DmsoDocument2 pagesat Bili DmsoP VijayaNo ratings yet
- Total Protein Test ProcedureDocument2 pagesTotal Protein Test ProcedureTrần Thanh ViệnNo ratings yet
- Sodium: Enzymatic MethodDocument2 pagesSodium: Enzymatic MethodFariz KasyidiNo ratings yet
- PI e BIL - DIRECT 15Document2 pagesPI e BIL - DIRECT 15Nia HidmahNo ratings yet
- γ-GT Determination Kinetic MethodDocument4 pagesγ-GT Determination Kinetic MethodpixiedustNo ratings yet
- 01 Glucose G71245R04Document7 pages01 Glucose G71245R04chem.rajavithiNo ratings yet
- Alat (GPT) Fs : Order Information Warnings and PrecautionsDocument4 pagesAlat (GPT) Fs : Order Information Warnings and Precautionssovi haswindhaNo ratings yet
- GGT FluitestDocument4 pagesGGT FluitestCristian LaraNo ratings yet
- PI e GLUC - GLUCDH 7Document2 pagesPI e GLUC - GLUCDH 7erlan murtiadiNo ratings yet
- COD reagents measure protein concentrationDocument1 pageCOD reagents measure protein concentrationNisa Javadd100% (2)
- Iga2 2017-06 v11Document5 pagesIga2 2017-06 v11Aliel BertránNo ratings yet
- Protap - Amylase (Dyasis)Document2 pagesProtap - Amylase (Dyasis)TEMPORALIS 2018No ratings yet
- PHP UYjhb 7Document2 pagesPHP UYjhb 7BerhaneNo ratings yet
- tu-apoa1Document1 pagetu-apoa1Nghi NguyenNo ratings yet
- 1105000I Rev. 02Document2 pages1105000I Rev. 02Riadh BenyoucefNo ratings yet
- BLOSR6X2010Document7 pagesBLOSR6X2010MustafaNo ratings yet
- PI e AST 2Document3 pagesPI e AST 2Cindy Mae A. PogoyNo ratings yet
- Ferritin Ing Rev. 03Document2 pagesFerritin Ing Rev. 03Yousra NanoNo ratings yet
- IFU - R910 e ALT 2Document4 pagesIFU - R910 e ALT 2Osama Ben DawNo ratings yet
- 11502I CreatinineDocument1 page11502I CreatininemahinNo ratings yet
- L.D.H. (LDH-P) : SFBC Modified MethodDocument2 pagesL.D.H. (LDH-P) : SFBC Modified MethodFariz KasyidiNo ratings yet
- PI e BIL - TOTAL 16Document2 pagesPI e BIL - TOTAL 16ilhamNo ratings yet
- GA4231 00 - Total BilirubinDocument2 pagesGA4231 00 - Total BilirubinTrần Thanh Viện100% (1)
- At 97553Document2 pagesAt 97553عبدالفتاح البكوشNo ratings yet
- Intended Use - Methodology - Reagents 1.Document6 pagesIntended Use - Methodology - Reagents 1.Dharmesh PatelNo ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2Document16 pagesLyphochek Assayed Chemistry Control Levels 1 and 2PATH LABNo ratings yet
- BioMed-Gamma GT 1+1Document2 pagesBioMed-Gamma GT 1+1BitolamNo ratings yet
- Lifespan Neurorehabilitation DNBDocument1,231 pagesLifespan Neurorehabilitation DNBShameem Ali100% (1)
- Scully 2016 PDFDocument14 pagesScully 2016 PDFDewi ZakiawatiNo ratings yet
- Case Study TrichoDocument6 pagesCase Study TrichoVisanth KumarNo ratings yet
- How to Care for Head InjuriesDocument31 pagesHow to Care for Head InjuriesZawawi Ibnu Rosyid0% (1)
- TabletsDocument2 pagesTabletsHector De VeraNo ratings yet
- Hema I Chapter 14 - CSFDocument28 pagesHema I Chapter 14 - CSFTesfaNo ratings yet
- Preventive Health Care and Corporate Female Workforce: ABBDocument8 pagesPreventive Health Care and Corporate Female Workforce: ABBnusrat_ahmad5805No ratings yet
- UPPER GASTROINTESTINAL BLEEDING Simp HemoroidDocument44 pagesUPPER GASTROINTESTINAL BLEEDING Simp HemoroidKomang YudaNo ratings yet
- GRAND PRES - CorrectedDocument22 pagesGRAND PRES - CorrectedDarlen RabanoNo ratings yet
- Clinical Value of Lead aVRDocument8 pagesClinical Value of Lead aVRmhelguera1No ratings yet
- Hospital Acquired InfectionsDocument51 pagesHospital Acquired Infectionstummalapalli venkateswara raoNo ratings yet
- Reticularis With Ulcerations: LivedoDocument21 pagesReticularis With Ulcerations: LivedoGabriela GheorgheNo ratings yet
- Toxicology Coverage Midterm Exam PDFDocument17 pagesToxicology Coverage Midterm Exam PDFNestor BargioNo ratings yet
- CataractsDocument17 pagesCataractsdubutwice29No ratings yet
- Letter From TGA DR Leonie Hunt To Balmoral Naval Hospital DR George Blackwood, 19 July 2000 PDFDocument3 pagesLetter From TGA DR Leonie Hunt To Balmoral Naval Hospital DR George Blackwood, 19 July 2000 PDFHenry BelotNo ratings yet
- Blood Donor Hematocrit Level InformationDocument1 pageBlood Donor Hematocrit Level Informationdoppler_No ratings yet
- DENTISTRY Assessment of Fundamental Knowledge 2014Document10 pagesDENTISTRY Assessment of Fundamental Knowledge 2014sarookaNo ratings yet
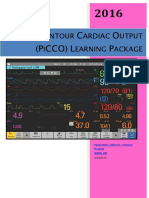
- Pulse Contour Cardiac Output Learning Package PDFDocument17 pagesPulse Contour Cardiac Output Learning Package PDFNurse PractitionerNo ratings yet
- First Aid Quiz SampleDocument11 pagesFirst Aid Quiz SampleKah WaiNo ratings yet
- Infeksi parasit di saluran cerna: Diagnosis dan pengobatan protozoa dan helminta penyebab diareDocument69 pagesInfeksi parasit di saluran cerna: Diagnosis dan pengobatan protozoa dan helminta penyebab diareRangga DarmawanNo ratings yet
- Idiophatic Thrombocytopenic Purpura (ITP) in PregnancyDocument27 pagesIdiophatic Thrombocytopenic Purpura (ITP) in PregnancyAdietya Bima PrakasaNo ratings yet
- Continue Renal Replacement Therapy: 1. CVVH 2. CVVHD 3. CVVHDFDocument21 pagesContinue Renal Replacement Therapy: 1. CVVH 2. CVVHD 3. CVVHDFhengki jokteryNo ratings yet
- Hyperemesis GravidarumDocument9 pagesHyperemesis GravidarumChyank Qu SaddamNo ratings yet
- Urodynamics: Committee 7Document56 pagesUrodynamics: Committee 7Coral Garcia RiveraNo ratings yet
- Sexual history and STI examDocument2 pagesSexual history and STI examLizaEllaga67% (3)
- (2016) Small Animal Abdominal Ultrasonography Liver & GallBladder - Part 1Document7 pages(2016) Small Animal Abdominal Ultrasonography Liver & GallBladder - Part 1ludiegues752100% (1)
- Dr. Naitik Trivedi & Dr. Upama Trivedi: Haemopoietic SystemDocument22 pagesDr. Naitik Trivedi & Dr. Upama Trivedi: Haemopoietic SystemthiruklpNo ratings yet
- Case Study - Depression PDFDocument6 pagesCase Study - Depression PDFxiejie22590No ratings yet
- Human Immunodeficiency Virus (HIV)Document34 pagesHuman Immunodeficiency Virus (HIV)Ahmed SafaNo ratings yet
- Berglundh Et Al-2018-Journal of PeriodontologyDocument6 pagesBerglundh Et Al-2018-Journal of PeriodontologyestefyNo ratings yet