Professional Documents
Culture Documents
Allotropes of Iron
Uploaded by
Karthik RangarajOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Allotropes of Iron
Uploaded by
Karthik RangarajCopyright:
Available Formats
Allotropes of iron
From Wikipedia, the free encyclopedia
Main article: Iron
Low-pressure phase diagram of pure iron. BCC is body centered cubic and FCC is face centered cubic.
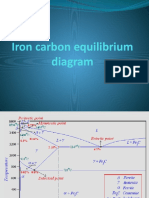
Iron-carbon eutectic phase diagram, showing various forms of FexCy substances.
Iron allotropes, showing the differences in lattice structure. The alpha iron (α) is a body-centered cubic (BCC)
and the gamma iron (γ) is a face-centered cubic (FCC).
Iron represents perhaps the best-known example for allotropy in a metal. At atmospheric pressure,
there are three allotropic forms of iron: alpha iron (α) a.k.a. ferrite, gamma iron (γ) a.k.a. austenite,
and delta iron (δ). At very high pressure, a fourth form exists, called epsilon iron (ε) hexaferrum.
Some controversial experimental evidence exists for another high-pressure form that is stable at
very high pressures and temperatures.[1]
The phases of iron at atmospheric pressure are important because of the differences in solubility
of carbon, forming different types of steel. The high-pressure phases of iron are important as models
for the solid parts of planetary cores. The inner core of the Earth is generally assumed to consist
essentially of a crystalline iron-nickel alloy with ε structure.[2][3][4] The outer core surrounding the solid
inner core is believed to be composed of liquid iron mixed with nickel and trace amounts of lighter
elements.
Contents
[hide]
1Standard pressure allotropes
o 1.1Delta iron (δ-Fe)
o 1.2Gamma iron / Austenite(γ-Fe)
o 1.3Beta iron (β-Fe)
o 1.4Alpha iron / Ferrite (α-Fe)
2High pressure allotropes
o 2.1Epsilon iron / Hexaferrum (ε-Fe)
o 2.2Experimental high temperature and pressure
3See also
4References
Standard pressure allotropes[edit]
Delta iron (δ-Fe)[edit]
As molten iron cools down, it solidifies at 1,595 °C (2,800 °F) into its δ allotrope, which has a body-
centered cubic(BCC) crystal structure.[5] δ-iron can dissolve as much as 0.09% of carbon by mass at
1,493 °C.
Gamma iron / Austenite(γ-Fe)[edit]
Main article: Austenite
As the iron cools further to 1,394 °C its crystal structure changes to a face centered cubic (FCC)
crystalline structure. In this form it is called gamma iron (γ-Fe) or Austenite. γ-iron can dissolve
considerably more carbon (as much as 2.04% by mass at 1,146 °C). This γ form of carbon
saturation is exhibited in stainless steel.
Beta iron (β-Fe)[edit]
Main article: beta iron
Beta ferrite (β-Fe) and beta iron (β-iron) are obsolete terms for the paramagnetic form of ferrite (α-
Fe).[6][7] The primary phase of low-carbon or mild steel and most cast irons at room temperature
is ferromagnetic ferrite (α-Fe). As iron or ferritic steel is heated above the critical
temperature A2 or Curie temperature of 771 °C (1044K or 1420 °F),[8] the random thermal agitation of
the atoms exceeds the oriented magnetic moment of the unpaired electron spins in the 3d
shell.[9] The A2 forms the low-temperature boundary of the beta iron field in the phase diagram in
Figure 1. Beta ferrite is crystallographically identical to alpha ferrite, except for magnetic
domains and the expanded body-centered cubic lattice parameter as a function of temperature, and
is therefore of only minor importance in steel heat treating. For this reason, the beta "phase" is not
usually considered a distinct phase but merely the high-temperature end of the alpha phase field.
Alpha iron / Ferrite (α-Fe)[edit]
Main article: Ferrite (iron)
At 912 °C (1,674 °F) the crystal structure again becomes BCC as α-iron is formed. The substance
assumes a paramagnetic property. α-iron can dissolve only a small concentration of carbon (no
more than 0.021% by mass at 910 °C).
At 770 °C (1,418 °F), the Curie point (TC), the iron is a fairly soft metal and becomes ferromagnetic.
As the iron passes through the Curie temperature there is no change in crystalline structure, but
there is a change in the magnetic properties as the magnetic domains become aligned. This is the
stable form of iron at room temperature.
High pressure allotropes[edit]
Epsilon iron / Hexaferrum (ε-Fe)[edit]
Main article: Hexaferrum
At pressures above approximately 10 GPa and temperatures of a few hundred kelvin or less, α-iron
changes into a hexagonal close-packed (hcp) structure, which is also known as ε-iron or
hexaferrum;[10] the higher-temperature γ-phase also changes into ε-iron, but does so at a higher
pressure. Antiferromagnetism in alloys of epsilon-Fe with Mn, Os and Ru has been observed.[11]
Experimental high temperature and pressure[edit]
An alternate stable form, if it exists, may appear at pressures of at least 50 GPa and temperatures of
at least 1,500 K; it has been thought to have an orthorhombic or a double hcp structure.[1] as of
December 2011, recent and ongoing experiments are being conducted on high-pressure
and Superdense carbon allotropes.
You might also like
- Moles Escape Room AnswersDocument4 pagesMoles Escape Room Answersapi-551132697No ratings yet
- Allotropes of IronDocument5 pagesAllotropes of IronShamsul RamliNo ratings yet
- Allotropes of IronDocument5 pagesAllotropes of IronVysakh VasudevanNo ratings yet
- Allotropes of IronDocument3 pagesAllotropes of Ironravi2007No ratings yet
- Iron Carbon Equilibrium DiagramDocument4 pagesIron Carbon Equilibrium DiagramParameshwari PrabakarNo ratings yet
- FMP 221 Lecture 4Document22 pagesFMP 221 Lecture 4SarojKumarSinghNo ratings yet
- Introduction-Iron Carbon Phase DiagramDocument31 pagesIntroduction-Iron Carbon Phase DiagramTHE BBEASTNo ratings yet
- Ferrite, also known as α-ferrite (α-Fe) or alpha iron, is a: strain fieldDocument2 pagesFerrite, also known as α-ferrite (α-Fe) or alpha iron, is a: strain fieldaqhammamNo ratings yet
- Lesson 5 - Fe-C Diagram - Rev. 0Document11 pagesLesson 5 - Fe-C Diagram - Rev. 0Arga SetyaNo ratings yet
- Unit Cell Cubic StructuresDocument8 pagesUnit Cell Cubic StructuresGuilherme Dos Santos MoreiraNo ratings yet
- Materials Engineering and Technology (MEE1005) Digital Assignment - IIDocument4 pagesMaterials Engineering and Technology (MEE1005) Digital Assignment - IIAmit ManthekarNo ratings yet
- Engineering Material II Short NoteDocument17 pagesEngineering Material II Short NotewondimuNo ratings yet
- Steel: Steel Is An Alloy of Iron and Carbon, and Sometimes Other Elements. Because of Its HighDocument15 pagesSteel: Steel Is An Alloy of Iron and Carbon, and Sometimes Other Elements. Because of Its HighRam TejaNo ratings yet
- The Iron-Carbon Phase DiagramDocument16 pagesThe Iron-Carbon Phase DiagramMeena SivasubramanianNo ratings yet
- Phase Diagrams:: The Iron-Iron Carbide (Fe-Fe3C) Diagram or Iron-Carbon (Fe-C) Equilibrium DiagramDocument46 pagesPhase Diagrams:: The Iron-Iron Carbide (Fe-Fe3C) Diagram or Iron-Carbon (Fe-C) Equilibrium DiagramUsman FarooqNo ratings yet
- Iron-Carbon DiagramDocument11 pagesIron-Carbon DiagramrampradNo ratings yet
- Iron Carbon Equillibrium Diagram GandhidhamDocument22 pagesIron Carbon Equillibrium Diagram Gandhidhamcal2_uniNo ratings yet
- Steel - WikipediaDocument15 pagesSteel - WikipediaARIF AHAMEDNo ratings yet
- Steel - WikipediaDocument16 pagesSteel - WikipediaaravindNo ratings yet
- Steel: From Wikipedia, The Free EncyclopediaDocument11 pagesSteel: From Wikipedia, The Free Encyclopediasatish_trivediNo ratings yet
- Bower Boiler SteelsDocument3 pagesBower Boiler Steelsraut_1234100% (1)
- The Metallurgy of Power BoilersDocument2 pagesThe Metallurgy of Power Boilersdineshkbunker08No ratings yet
- Metallury of SteelsDocument10 pagesMetallury of SteelsDalitso MwanzaNo ratings yet
- SteelDocument19 pagesSteelEdi YantoNo ratings yet
- The Iron Carbon Equilibrium DiagramDocument7 pagesThe Iron Carbon Equilibrium DiagrambalajiNo ratings yet
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument52 pagesCh-27.5 Iron Carbon Equilibrium DiagramManojNo ratings yet
- Composition of Plain Carbon Steel Carbon Steel or Plain-Carbon Steel, Is A Metal Alloy. It Is A Combination ofDocument14 pagesComposition of Plain Carbon Steel Carbon Steel or Plain-Carbon Steel, Is A Metal Alloy. It Is A Combination ofkayodeNo ratings yet
- Ch-27.3 Iron Carbon Equilibrium DiagramDocument58 pagesCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfNo ratings yet
- IronDocument23 pagesIronEdi YantoNo ratings yet
- SteelDocument8 pagesSteelvivek463No ratings yet
- Definitions and Related Materials: Stahliją or Stakhlijan (Made of Steel), Stahliją (Standing Firm)Document5 pagesDefinitions and Related Materials: Stahliją or Stakhlijan (Made of Steel), Stahliją (Standing Firm)kingNo ratings yet
- Steel: Members: Benz Andrew Regis Jefferson Carido Sheila Mae CanilloDocument30 pagesSteel: Members: Benz Andrew Regis Jefferson Carido Sheila Mae CanilloBenz Andrew RegisNo ratings yet
- Iron - WikipediaDocument20 pagesIron - Wikipediaramthecharm_46098467No ratings yet
- Iron-Carbon DiagramDocument3 pagesIron-Carbon DiagramnaniNo ratings yet
- Steel: Navigation SearchDocument15 pagesSteel: Navigation Searchamol_khedkar_2No ratings yet
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 pagesUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongNo ratings yet
- Austenite: Iron-carbon phase diagram, showing the conditions under which austenite (γ) is stable in carbon steelDocument4 pagesAustenite: Iron-carbon phase diagram, showing the conditions under which austenite (γ) is stable in carbon steelVysakh VasudevanNo ratings yet
- Constr Materials B PDFDocument72 pagesConstr Materials B PDFAgniva DuttaNo ratings yet
- Metallurgy - Chapter (5) - Steels and Cast IronsDocument79 pagesMetallurgy - Chapter (5) - Steels and Cast IronsKarim Mamdouh100% (1)
- Engineering Metallurgy: Misan University-College of EngineeringDocument27 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manNo ratings yet
- Metastable Iron-Carbon (Fe-C) Phase DiagramDocument3 pagesMetastable Iron-Carbon (Fe-C) Phase DiagramupenderNo ratings yet
- MEC 414 - Iron Phase Diagram Experiment 2Document7 pagesMEC 414 - Iron Phase Diagram Experiment 2boatcomNo ratings yet
- Structure of Plain SteelDocument4 pagesStructure of Plain Steelsatish_trivediNo ratings yet
- The IronCarbide DiagramDocument11 pagesThe IronCarbide DiagramshajjikhalidNo ratings yet
- Heat Treatment of Cast IronsDocument4 pagesHeat Treatment of Cast IronshamidrezachamaniNo ratings yet
- Analisis Sifat Mekanik Baja SKD 61 Dengan Baja ST 41 Dilakukan Hardening Dengan Variasi TemperaturDocument11 pagesAnalisis Sifat Mekanik Baja SKD 61 Dengan Baja ST 41 Dilakukan Hardening Dengan Variasi TemperaturFarhan WartiansyahNo ratings yet
- Engineering Materials-Chapter One-Ferrous Alloys HighlightedDocument32 pagesEngineering Materials-Chapter One-Ferrous Alloys HighlightedMohammed AssadNo ratings yet
- Steel: Iron AlloyDocument14 pagesSteel: Iron AlloySiva BhaskarNo ratings yet
- Engineering Material - : Chapter TwoDocument41 pagesEngineering Material - : Chapter TwoAla ZiNo ratings yet
- The National Board of Boiler and Pressure Vessel InspectorsDocument3 pagesThe National Board of Boiler and Pressure Vessel Inspectorsmiguel arandaNo ratings yet
- The Iron-Carbon Equilibrium Diagram: AbstractDocument4 pagesThe Iron-Carbon Equilibrium Diagram: AbstractRama Krishna Reddy DonthireddyNo ratings yet
- Metallurgy AssignmentDocument9 pagesMetallurgy Assignmentvishnupriya somaganiNo ratings yet
- Metallurgy AssignmentDocument9 pagesMetallurgy Assignmentvishnupriya somaganiNo ratings yet
- The Iron-Carbon Equilibrium Diagram: AbstractDocument4 pagesThe Iron-Carbon Equilibrium Diagram: Abstractleodavid87No ratings yet
- What Is Iron? What Is Steel?Document4 pagesWhat Is Iron? What Is Steel?CiberNo ratings yet
- Iron Carbon Equilibrium DiagramDocument52 pagesIron Carbon Equilibrium DiagramSohan Lal100% (2)
- MetallurgyDocument190 pagesMetallurgyJose J. Nuñez100% (2)
- Steels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceDocument39 pagesSteels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceKareem YasserNo ratings yet
- Steel CompositionDocument8 pagesSteel CompositionShahab28No ratings yet
- Cast Steel: The Iron-Carbon Equilibrium Diagram: AbstractDocument5 pagesCast Steel: The Iron-Carbon Equilibrium Diagram: Abstractchacha4500No ratings yet
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelFrom EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo ratings yet
- Tenancy Agreement ﺭﺎــــــﺠـﻳﺇ ﺪـﻘــــــﻋDocument2 pagesTenancy Agreement ﺭﺎــــــﺠـﻳﺇ ﺪـﻘــــــﻋKarthik RangarajNo ratings yet
- Resume R. Karthik: Key Work ExperienceDocument5 pagesResume R. Karthik: Key Work ExperienceKarthik RangarajNo ratings yet
- Kayakalpa PayirchiDocument15 pagesKayakalpa PayirchiKarthik RangarajNo ratings yet
- 10.MER-QP-010 Rev.2 Monitoring ActivitiesDocument3 pages10.MER-QP-010 Rev.2 Monitoring ActivitiesKarthik RangarajNo ratings yet
- S BlockDocument27 pagesS BlockAditya BansalNo ratings yet
- Science7 q1 Mod2 Elementsandcompoundspart1 1-22Document22 pagesScience7 q1 Mod2 Elementsandcompoundspart1 1-22api-114144039No ratings yet
- VC & VF PDFDocument1 pageVC & VF PDFPuneeth KumarNo ratings yet
- Nový Kód Starý Kód Moc S DPH Odporúčaná Cena: Žiarovky, Pätice, MateriálDocument53 pagesNový Kód Starý Kód Moc S DPH Odporúčaná Cena: Žiarovky, Pätice, MateriálShadi AbdelsalamNo ratings yet
- An Analysis of Late Pre Islamic Copper BDocument13 pagesAn Analysis of Late Pre Islamic Copper Babdelrahman elgawish (Dr)No ratings yet
- Practicum Group 2 The Metal CharacteristicDocument8 pagesPracticum Group 2 The Metal CharacteristicLamtiur SilabanNo ratings yet
- Mendeleev PT ActivityDocument6 pagesMendeleev PT ActivityLaura PoloNo ratings yet
- General Chemistry 2Document39 pagesGeneral Chemistry 2Lhor MaceroNo ratings yet
- Chemistry March 2019 STD 12th Science HSC Maharashtra Board Question PaperDocument4 pagesChemistry March 2019 STD 12th Science HSC Maharashtra Board Question PaperDeadshotNo ratings yet
- Binary Phase Diagrams For Selected Platinum Alloys: Supplementary DatasheetDocument2 pagesBinary Phase Diagrams For Selected Platinum Alloys: Supplementary DatasheetJulio GonzalezNo ratings yet
- MCX Lot SizeDocument6 pagesMCX Lot SizeVt ShivaNo ratings yet
- JEE Advanced 2022 Solved Paper 1Document12 pagesJEE Advanced 2022 Solved Paper 1Gaurav KumarNo ratings yet
- Types of Decomposition ReactionDocument19 pagesTypes of Decomposition ReactionSrynnENo ratings yet
- Data XRF ST14Document78 pagesData XRF ST14Achmad Nabil ZulfaqarNo ratings yet
- Bronze E-BrochureDocument32 pagesBronze E-BrochureRizky MahendraNo ratings yet
- Analysis Presentation 1Document12 pagesAnalysis Presentation 1Muhammad Hussain KhalilNo ratings yet
- Unit 37Document122 pagesUnit 374C06 Cheng Sum Yi ZitaNo ratings yet
- MCQ Questions For Class 10 Science CH - 2 Acids, Bases, and SaltsDocument12 pagesMCQ Questions For Class 10 Science CH - 2 Acids, Bases, and SaltsMiss none of your businessNo ratings yet
- 1.1 PowerofamagnetDocument20 pages1.1 PowerofamagnetEngelo CaroNo ratings yet
- WFP Iodised SaltDocument3 pagesWFP Iodised SaltMarcel NKWEKAMNo ratings yet
- The Pauli Exclusion PrincipleDocument33 pagesThe Pauli Exclusion Principle3449336893No ratings yet
- U15 S4 HW Packet 13-20Document27 pagesU15 S4 HW Packet 13-20Rohith GudatiNo ratings yet
- Organic Compounds Inorganic CompoundsDocument42 pagesOrganic Compounds Inorganic CompoundsBabar NaseerNo ratings yet
- NMSI Notes - Chapter 17 PDFDocument8 pagesNMSI Notes - Chapter 17 PDFmbugua simon ngigiNo ratings yet
- A New Method For The Synthesis of Aliphatic Nitro Compounds1, 2Document5 pagesA New Method For The Synthesis of Aliphatic Nitro Compounds1, 2banjo01No ratings yet
- Trial 2023 p4Document11 pagesTrial 2023 p4Marco MedhatNo ratings yet
- Reacting Mass Calculations 1: © WWW - CHEMSHEETS.co - Uk 24-October-2016 Chemsheets GCSE 1093Document2 pagesReacting Mass Calculations 1: © WWW - CHEMSHEETS.co - Uk 24-October-2016 Chemsheets GCSE 1093George AshcroftNo ratings yet
- Iso 439 1994 PDFDocument9 pagesIso 439 1994 PDFdglpssenthilkumar497No ratings yet
- Mock Engineering MaterialsDocument6 pagesMock Engineering MaterialsJohn AsokNo ratings yet