Professional Documents
Culture Documents
42d AP Second Thermodynamics
Uploaded by
diegoCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
42d AP Second Thermodynamics
Uploaded by
diegoCopyright:
Available Formats
Lesson 42d: Second Law of Thermodynamics & Entropy
The Second Law of Thermodynamics
If you take a can of Dr Pepper out of the fridge and walk outside on a hot summer day, you expect the
drink to get warmer as time passes. You would never expect it to get colder.
• This is what the second law of thermodynamics is all about. If two objects at different
temperatures come in contact with each other, the hotter one will get colder as the colder one
gets hotter, until eventually they would be the same temperature.
• The second law of thermodynamics says that heat will always spontaneously flow from a
hotter to a colder object.
We must consider this law (along with the first law) to be able to explain things like heat engines,
refrigerators, and heat pumps.
Heat Engines Hot Reservoir
A heat engine uses energy to perform work, as shown in Illustration 1.
• The heat that is the input to run the engine comes from an area at a QH
higher temperature, called a hot reservoir.
• Some of the input is used to do some actual work. This is the output
that we actually want. engine W
• The rest of the heat is released (basically as waste) to a cold
reservoir.
QC
Notice how this obeys both the first and second laws.
• The input heat is equal to the work done plus the heat released
(first law). Cold Reservoir
Qh = W + Qc Illustration 1: A heat
• The heat is flowing from the hot to the cold reservoir (second law). engine.
An efficient heat engine should produce as much work as possible from the least input heat possible.
• To calculate the efficiency of a heat engine, we need to compare the work done to the input heat
by dividing them.
W
e=
Qh
e = efficiency (%)
W = Work done (J)
Qh = input heat from hot reservoir (J)
* N.B. All done as absolute values
Example 1: A automobile engine is able to do 2000 J of work to move a car. If the heat generated in
the engine from burning the gas is 9000 J, determine the efficiency of the engine.
W 2000
e= = =22.22 %
Qh 9000
5/5/2011 © studyphysics.ca Page 1 of 6 / C&J Section 15.7-15.11
• We can take our formula for efficiency and combine it with the formula we have from above for
the first law.
1st Law Formula...
Qh = W + Qc → W = Qh - Qc
combined with 2nd Law formula for efficiency...
W
e= We can divide each term on the top
Qh individually by Qh.
Q h−Q c
e=
Qh
Q Q To be able to solve for a formula in
e= h − c the next section, we need to write
Q h Qh
this formula this way. Trust me.
Qc
e=1−
Qh
• This version of the formula for the efficiency of a heat engine lets us do a calculation if we
know the input heat from the hot reservoir and the output heat to the cold reservoir.
Example 2: A heat engine is running with an input of heat of 1.45e4 J. If it dumps 1.02e4 J of waste
heat into its surroundings, determine the efficiency of the engine.
Q
e=1− c
Qh
1.02e4
e=1−
1.45e4
e=29.7 %
Carnot's Heat Engine
By the early 1800's physicists were asking themselves if there was a limit to how efficient an engine
could be.
• It may sound like an easy question, with an answer of 100%.
◦ Sadly, this is impossible, and Sadi Carnot is the man who proved it using his own way of
looking at the second law.
• According to Carnot, a heat engine reaches maximum efficiency if everything about it is
reversible.
◦ What he meant by reversible is that it would be possible for the engine itself(the system),
and everything around the engine (the surroundings) to return to exactly the same states they
were in before the engine was running.
▪ In real life this is most often impossible because of the effect of friction. The waste heat
that is released from friction can not be returned.
▪ Even without friction, many processes would not be reversible since heat would need to
flow on its own from cold to hot, and the second law says that can't happen.
◦ Still Carnot's ideas about heat engines are helpful,
since a Carnot Engine let us predict the best The second law can be said this
way.
theoretical efficiency of an engine in a particular
situation. We know that any real engine must have
an efficiency less than the Carnot engine in that same situation.
5/5/2011 © studyphysics.ca Page 2 of 6 / C&J Section 15.7-15.11
To figure this all out, Carnot focused on the temperatures of the hot and cold reservoirs of the heat
engine.
• He reasoned that the best possible engine would be one that did the most work based on the
temperature difference that it had available between the two reservoirs.
◦ Kind of think of it like we want the hottest hot reservoir possible to draw from, and the
coldest cold reservoir to dump in to. We hope this means a lot of heat will be available to
change into work.
• It can be shown that the ratio of the heat of the two reservoirs is equivalent to the ratio of their
temperatures.
Qc T c
=
Qh T h
*N.B. Temperatures must be measure in Kelvin.
• We can substitute this into the formula for efficiency that we came up with above.
Q
e=1− c
Qh
This is the efficiency of a Carnot
Tc heat engine as it appears on the
e c=1− data sheet. Remember to use Kelvin
Th
for the temperature.
T h−T c
ec=
Th
If you look at the second last line of formula substitution above, you might get an idea Tc
of why a 100% efficient engine is simply impossible. e c =1−
Th
Even in Carnot's perfectly reversible engine, there must be a difference in
temperature between the two reservoirs, so that heat can flow on its own (second
law). Since Tc < Th , we will always be subtracting some decimal from 1 in the
formula. That gives us an efficiency less that 100%.
Example 3: You want to set up a heat engine that attaches to your window in the winter time, so that it
uses the inside of your house at 22oC as the hot reservoir, and the outdoors at -18oC as the cold
reservoir. Determine the maximum efficiency of this engine. Also explain a drawback of this setup.
T −T c
e c= h
Th
295−255
ec=
295
ec =13.6 %
There are a couple of drawbacks that might be worth mentioning. First of all, 13.6% is not
exactly a great efficiency; there's probably a better way to get some work done.
The second reason goes against the common idea that this is still free work you're getting just
because your home is warmer than outside. You're forgetting that the heating system of your
home needs to use energy to heat your home. By using you heat engine on your window, you
force your home heating system to work harder to keep the temperature of the house up. Since
the heating system can not work at 100% efficiency, you are actually wasting more heat.
5/5/2011 © studyphysics.ca Page 3 of 6 / C&J Section 15.7-15.11
Refrigerators and Heat Pumps Hot Reservoir
Heat will never flow on its own (spontaneously) from cold to hot, but that
doesn't mean we can't force it to! Qh
• A device like a refrigerator is basically a heat engine that runs in
reverse, so that we do work to move heat from a cold reservoir to a
hot reservoir. fridge W
• For a fridge in your home, doing work to pump the heat out of cold
reservoir (inside the fridge) to the hot reservoir (the coils on the
back / bottom on the outside of the fridge) will make the inside colder Qc
and the outside (your kitchen) warmer.
• This still agrees with the 1st law, since Qh = W + Qc is still true.
• It also agrees with the 2nd law, since none of this happened Cold Reservoir
spontaneously. Illustration 2: A
refrigerator.
A heat pump is a device that works like a refrigerator,
except that it is used to heat a building in cold weather. Did You Know?
An air conditioner is basically the same as a
• For a heat pump, the cold reservoir is the refrigerator. The difference is that your entire
outdoors, and the hot reservoir is the inside of the house is the cold reservoir, and the outdoors
building. is the hot reservoir.
• Electricity is used to do the work of pumping the
heat.
Example 4: Explain why a heat pump is better for heating a home than a regular plug in space heater
from WalMart. Assume that you have 500 J of electrical energy to do the work of running either one.
Let's start with the regular space heater you bought for cheap at WalMart. It probably just has a
bunch of heating coils that get hot when you plug it in. We'll give it the benefit of the doubt and
say it is 100% efficient, so that all 500 J of electricity going in (the work) becomes 500 J of heat
for your home.
There is no cold reservoir for a
Qh = W + Qc space heater.
Qh = W + 0
Qh = W
Qh = 500 J
Now we look at the heat pump. It follows the same rules a refrigerator, so it is moving heat
from a cold reservoir to a hot reservoir. Because of this, Qc must be more than zero!
Qh = W + Qc If Qc is some number bigger than
Qh = 500 + Qc zero, Qh must be more than just
Qh > 500 J 500 J.
So the heat pump results in more heat being transferred to the room than just the 500 J from the
work done. Although this is great, the disadvantage is that a heat pump must be installed with
some sort of hose to the outside to use outdoors as a cold reservoir.
5/5/2011 © studyphysics.ca Page 4 of 6 / C&J Section 15.7-15.11
Example 5: For each of these diagrams, explain why the process shown is impossible.
a)
In this situation all of the heat from the hot reservoir is
100 J
being converted to work; no heat is going to the cold
100 J
reservoir. This violates the 2nd law, since heat flows from
hot to cold, and no system can be 100% efficient.
b)
100 J In this case we have 100 J of heat from the hot reservoir
50 J doing 50 J of work and 70 J of heat moving to the cold
reservoir. A quick check shows that this breaks the first
70 J law, since Qh = W + Qc → 100 ≠ 50 + 70
c)
100 J This one is acting as a refrigerator, which on its own is
85 J fine, but it's the numbers that are all screwed up. It breaks
the first law (sort of like the last example), since Qh = W +
20 J
Qc → 100 ≠ 85 + 20
d)
15 J Again, a refrigerator. This one looks like it might be fine
85 J because as 100 J goes into the engine, 100 J comes out (15
J to the hot reservoir and 85 J of work). The only problem
100 J is that for a fridge to function, the work has to be done on
the engine, not by the engine. This violates the 2nd law.
Entropy
In the real world, no matter what we do there is no such think as a perfect engine or fridge. Every
process is going to involve some friction and loss of usable energy.
• Even in a perfect situation with a Carnot engine, the temperature between the hot and cold
reservoirs will eventually become the same (thermal equilibrium) since the heat will always
flow from hot to cold.
◦ When we reach this point and Tc = Th, efficiency becomes zero according to the formula...
T
e c =1− c
Th
T
e c =1− h
Th
e c =1−1=0 %
5/5/2011 © studyphysics.ca Page 5 of 6 / C&J Section 15.7-15.11
• Eventually, some day in the very distant future, when all the possible processes have happened,
all the universe will be at the same temperature with absolutely no way for anything to do any
work any more.
◦ This is sometimes referred to as the “heat death of the universe,” or less dramatically
entropy.
Entropy is a measure of the disorder of a system.
• On their own, all systems tend towards an increase in entropy unless work is done on them.
• This is actually just a different way of stating the second law.
As an example, you accidentally push a vase off a table and it breaks when it hits the ground.
• This increases the disorder of the system.
◦ Although we could put it back together with glue and a bit of effort, this means that we
would have to do work on the system to decrease its entropy.
◦ If we left it alone, we would never expect the vase to glue itself back together. Although this
would not break the first law, it does violate the second law.
5/5/2011 © studyphysics.ca Page 6 of 6 / C&J Section 15.7-15.11
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Presentation HVACDocument45 pagesPresentation HVACMistyNo ratings yet
- Chiller Plant OptimizationDocument38 pagesChiller Plant OptimizationAnonymous 6hptH95100% (2)
- Sru Heat ExchangerDocument29 pagesSru Heat ExchangerJordan Young100% (1)
- Detailed Lesson PlanDocument7 pagesDetailed Lesson Planlenie bacalsoNo ratings yet
- Details of CondenserDocument7 pagesDetails of Condenseralwar_shi262068100% (3)
- Trevor M. Letcher Developments and Applications in Solubility 2007 PDFDocument433 pagesTrevor M. Letcher Developments and Applications in Solubility 2007 PDFmf1988No ratings yet
- Temperature ConverterDocument7 pagesTemperature ConverterAli Abdurrahman SungkarNo ratings yet
- ThermodynamicsDocument12 pagesThermodynamicsShailendera SinghNo ratings yet
- Orlander Text BookDocument783 pagesOrlander Text BookKai CaoNo ratings yet
- Basics of ThermodynamicsDocument39 pagesBasics of Thermodynamicssantoshgs191811No ratings yet
- Bimetal Thermometer Model 55, High-Quality Process Version Per EN 13190Document7 pagesBimetal Thermometer Model 55, High-Quality Process Version Per EN 13190Mai Thế ToanNo ratings yet
- UK Guide TX Home Oct 15Document36 pagesUK Guide TX Home Oct 15Pascariu FlorinNo ratings yet
- FTPDocument6 pagesFTPGabi BarzăNo ratings yet
- Mechanical Engineering Objective Type (10000+ Objective Questions) by D.handa, H.B.keswani BookDocument534 pagesMechanical Engineering Objective Type (10000+ Objective Questions) by D.handa, H.B.keswani BookPrakash RajNo ratings yet
- Basics of MechDocument31 pagesBasics of MechAgateNo ratings yet
- Cop and EerDocument3 pagesCop and Eerطاہر رضاNo ratings yet
- Rezistenta Termica Perete ParterDocument4 pagesRezistenta Termica Perete ParterStelian BerceaNo ratings yet
- National HVAC Design Report ENERGY STAR Certified Homes Rev10Document4 pagesNational HVAC Design Report ENERGY STAR Certified Homes Rev10thamerkayedNo ratings yet
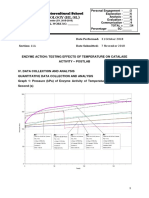
- Enzyme Activity - PostlabDocument9 pagesEnzyme Activity - PostlabDiva AderlyNo ratings yet
- Air Conditioning 1Document23 pagesAir Conditioning 1Cornel IordacheNo ratings yet
- Air Conditioning Unit With Remote Air Cooled Condenser: Working ConditionsDocument2 pagesAir Conditioning Unit With Remote Air Cooled Condenser: Working ConditionsJulianto zhengNo ratings yet
- Thermodynamics 1 by Sta. Maria Chapter 3 Solution ManualDocument7 pagesThermodynamics 1 by Sta. Maria Chapter 3 Solution ManualAllen MalabarbasNo ratings yet
- Theories in The Evolution of Chemical Equilibrium: Impli-Cations For Its Teaching and LearningDocument14 pagesTheories in The Evolution of Chemical Equilibrium: Impli-Cations For Its Teaching and LearningSebastián Cárdenas QuiñonesNo ratings yet
- Data Logger.Document10 pagesData Logger.diela9026No ratings yet
- VHRDocument2 pagesVHRGenevieve PaulNo ratings yet
- Pre-IG 0.13 Sep Mid-Term Physics - 1Document12 pagesPre-IG 0.13 Sep Mid-Term Physics - 1Kaung Khant ZawNo ratings yet
- 冷氣天書Document12 pages冷氣天書alberfel.lkNo ratings yet
- MathforRealLife PDFDocument214 pagesMathforRealLife PDFNarasimha Sandaka100% (1)
- Molecules 22 01186 v3Document22 pagesMolecules 22 01186 v3d crosbieNo ratings yet
- MEP 4th Ed 2019 Worked Sols Chap 23Document12 pagesMEP 4th Ed 2019 Worked Sols Chap 23Kenneth JameroNo ratings yet