Professional Documents
Culture Documents
Comparison of Surface Roughness Plaque Retention
Uploaded by
Martin AdriazolaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Comparison of Surface Roughness Plaque Retention
Uploaded by
Martin AdriazolaCopyright:
Available Formats
Dent Mater 13:258-269, July, 1997
Comparison of surface roughness of
oral hard materials to the threshold surface
roughness for bacterial plaque retention:
A review of the literature
C u r d M. L. B o l l e n l , 2, P a u l L a m b r e c h t s 3, M a r c Q u i r y n e n 1,2
1Department of Periodontology, School of Dentistry, Oral Pathology and Maxillo-facial Surgery,
Catholic University of Leuven, Leuven, BELGIUM
~Research Group for Microbial Adhesion, Leuven, BELGIUM
3BIOMAT, Department of Operative Dentistry and Dental Materials, School of Dentistry,
Oral Pathology and Maxillo-Facial Surgery, Catholic University of Leuven, Leuven, BELGIUM
ABSTRACT INTRODUCTION
Objectives. The roughness of intraoral hard surfaces can influence The oral cavity is constantly c o n t a m i n a t e d by m a n y
bacterial plague retention, The present review evaluates the initial diverse microbial species. Most of these microorganisms,
surface roughness of several intraoral hard materials, as well as changes especially those which are responsible for caries (e.g.,
in this surface roughness as a consequence of different treatment Streptococcus mutans a n d Lactobacillus spp.) a n d
modalities. periodontitis (e.g.,Actinobacillus actinomycetemcomitans
Methods. Articles found through Medtine searches were included in this and Porphyromonas gingivalis), can only survive in the
review if they met the following criteria: 1) stated threshold surface mouth when they adhere to non-shedding surfaces. The
roughness values and reputed change in surface roughness due to surface roughness of intraoral hard surfaces is of clinical
different manipulation techniques; or 2) included standardized surface importance in the process of bacterial retention. Changes
conditions that could be compared to the treated surface. in this variable might, therefore, facilitate the prevention
Results. Recently, some in vivo studies suggested a threshold surface of caries and periodontitis. The surface free energy can
roughness for bacterial retention (R, = 0.2 pro) below which no further also play a role in bacterial adhesion and retention;
reduction in bacterial accumulation could be expected. An increase in however, several studies (e.g., Q u i r y n e n et al., 1990;
surface roughness above this threshold roughness, however, resulted in Quirynen and BoUen, 1995) suggested that the influence
a simultaneous increase in plaque accumulation, thereby increasing the of the surface roughness overrules the influence of the
risk for both caries and periodontal inflammation. The initial surface surface free energy.
roughness of different dental materials (e.g., teeth, abutments, gold, Studies by Quirynen et al. (1990) showed t h a t an
amalgam, acrylic resin, resin composite, glass ionomer or compomer increase in the surface roughness of resin strips above an
and ceramics) and the effect of different treatment modalities (e.g., R a value of 2 pm resulted in a dramatic increase in the
polishing, scaling, brushing, condensing, glazing or finishing) on this bacterial colonization of these surfaces in comparison to
initial surface roughness were analyzed and compared to the threshold smooth strips (R, = 0.12 ~ra), whereas a change in the
surface roughness of 0.2 pro. The microbiological effects of these surface free energy had almost no impact. Recent studies
treatment modalities, if reported, are also discussed and compared to on abutments of intraoral two-stage implants (Quirynen
recent in vivo data. et al., 1996; Bollen et al., 1996) indicated that an increase
Significance. Based on this review, the range in surface roughness of in surface roughness (up to 0.8 pm) of these intraoral hard
different intraoral hard surfaces was found to be wide, and the impact of surfaces had a significant effect on the in vivo rate of plaque
dental treatments on the surface roughness is material-dependent. Some formation (supra- and subgingivally) only if the initial
clinical techniques result in a very smooth surface (compressing of surface had a minimum R~ value of 0.2 p m (see profile
composites against matrices), whereas others made the surface rather images, Fig. 1). Therefore, a "threshold R a" was suggested,
rough (application of hand instruments on gold). These findings which can be located at an R, score of 0.2 pm.
indicated that every dental material needs its own treatment modality in This R~ score is supported by the theory of bacterial
order to obtain and maintain a surface as smooth as possible. adhesion and retention. Physically, bacterial adhesion and
258 Bollen et aL/Surface roughness of oral hard materials: A review
MATERIALS AND METHODS
ROUGH
The articles included in this review were selected using a
(R, - 0 8[ p.rn) lJ . ,, Medline search (U.S. National Library of Medicine). The
inclusion criterion was the presence of data on surface
roughness. The following i n t r a o r a l m a t e r i a l s were
studied: natural teeth (9 studies), impl.ant abutments
(5 studies), amalgam (8 studies), gold (6 studies), resin
composites (17 studies), acrylic resin (7 studies), glass
ionomers and compomers (3 studies), a n d ceramics
(4 studies). If microbiological changes in relation to the
0.8 p m q r J roughness were available, they were included.
ST RESULTS
(R, = 0.21 ,~rn)
Tooth: E n a m e l a n d d e n t i n / cementum. T h e mean R a
values, before and after professional therapies, are depicted
-1.2 prn J
in Table 1. Von Mierau et al. (1982) measured the surface
roughness in vivo of crowns of natural teeth in 16 patients
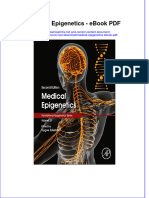
Fig. 1. Profile images of 2 test abutments over the same assessment length (ROUGH using a specially designed roughness probe. They reported
= roughened standard abutment, ST -- standard abutment). The length of the that roughness depended on the tooth type (canines were
profiles is 0.12 pm. The surface roughness (R: arithmetic mean of the departures the roughest) and on the location in the oral cavity (teeth
of the profile from the mean line) was measured with the Talysurf (Taylor-Hobson, in the upper jaw were rougher). All Ra values were above
Leicester, England).
3.5 ~m.
The effect of scaling on roots was examined by Rosenberg
retention occur in four phases: transport of the bacterium and Ash (1974). Fifty-eight teeth were divided into three
toward the surface, initial bacterial adhesion, attachment groups before extraction: 20 teeth were scaled by curettes,
by specific interactions, and finally, colonization of the 20 teeth were scaled by cavitron and the remaining 18
surfaces (Quirynen and Bollen, 1995). Initial bacterial teeth served as controls. The initial surface roughness of
adhesion and retention are physico-chemically possible 18.30 ~m decreased after curette scaling (to 9.51 ~m),
because a bacterium and a surface interact with each other whereas the cavitron scaling had no significant effect on
from a certain distance (approximately 50 nm) through a the tooth roughness (17.21 pm). In an earlier study, Green
combination of van der Waal's attractive forces and and Rami]ord (1966) found almost t h e same results
electrostatic repulsive forces. Lekness and Lie (1991) reported that the scaling of roots
Preferential retention occurs on rough surfaces since followed by polishing with chalk and pumice could decrease
bacteria on such surfaces are more protected against shear the surface roughness to nearly 1.3 ~m, which is still high
forces and can, thereby, have the necessary time to reach compared to the 0.2 pm threshold roughness.
direct contact or to bridge the distance. Initial colonization Oral hygiene implements have almost no influence on
of enamel surfaces was indeed shown to start from surface the initial roughness of crowns. Smith et al. (1986), as
irregularities (e.g., pits, grooves or abrasion defects) where well as Slop a n d A r e n d s (1987), s h o w e d t h a t t h e
bacteria are strongly protected (Nyvad and Fejerskov, application of floss or toothbrushes did not change the R~
1987). At these locations, the attachment may be more value, whereas the use of wooden toothpicks resulted in
s t r o n g l y e s t a b l i s h e d ( Q u i r y n e n a n d Bollen, 1995). an increase in the surface roughness. The difference of
Furthermore, several studies stated that proliferation of the latter roughness values from the R~ values reported
the initially adhering microorganisms accounts for the by Von Mierau et al. (1982) can be explained by the
major part of the microbial mass increase during early different device that was used to measure the surface
plaque formation (Brecx et al., 1983) which may explain roughness. Whereas Slop and Arends (1987) and Smith
the importance of surface roughness in initial plaque et al. (1986) used a computer- guided profilometer on
formation. extracted teeth, Von Mierau et al. (1982) had to use a
Subgingivally, the impact of surface roughness is much manual probe for measuring the surface roughness on teeth~
smaller. The pocket by itself offers a shelter, which limits in the oral cavity.
t h e p o s s i b l e i m p a c t of s u r f a c e r o u g h n e s s on t h e Polishing teeth has different effects on roughness,
subgingival plaque composition. Moreover, different means depending on the paste used. A recent study (Lutz et al.,
are available in this environment for bacterial survival: 1995) showed that polishing dentin (with an initial R of
adhesion to root cementum, adhesion to the desquamat- 0.03 pm) with different materials resulted in an increase
ing pocket epithelium, immersion in the crevicular fluid, in roughness up to 0.131 ~m (pumice). Only the application
invasion of the soft tissue and invasion of the hard tissue of CCS 40 (Clean Chemical AB, Upplands V~isby, Sweden)
via the dentin tubuli (Quirynen and Bollen, 1995). stabilized the R value at 0.03 pm. When enamel (initially
The aim of this literature review was to compare the 0.03 ~m) was polished, only pumice led to an increase in
surface roughness of different materials and the change roughness (up to 0.16 pm); none of the other materials
!n surface roughness by different manipulation techniques influenced the R value (Table 1).
m relation to the stated threshold surface roughness of No studies concerning microbiological effects and
0.2 ~m. results were available.
Dental Materials~July 1997 259
inflammatory and immune events in
the peri-implant mucosa and the gin-
giva around natural teeth are similar.
Therefore, the observations made of
artificial abutments can be extrapo-
Green and Ramfjord, 1966 Scaling (sickles) 9.12 + 2.67 lated to the root surfaces of natural
Scaling (curettes) 10.05 + 2.29 teeth.
Scaling (files & curettes) 10.54 + 2.45 When six commercially available
Scaling (hoes) 12.89 + 2.16
systems were compared, only the Steri-
ScalinQ (files) ~ 13.95 ± 3.69
" " " "~ " "" ": ""~ "~" " ~i. ~- Oss (Denar Corp.,Anaheim, CA, USA)
"
and the IMZ abutments (AG Sparte
Heath and Wilson, 1976 Brushing (Enamel) 0.10" Medizin Technik, Mannheim,
Polishing (rubber cup) 0.50* Germany) seemed to have an Ra value
Finishing (white stone) 0.50* below 0.2 pm (Quirynen et al., 1994).
Polishing (prophylactic paste) 0.80* Recently it was shown that electro-
Polishing (Zr discs) 0.80* and mechano-polishing of standard
Brtmemark abutments (Nobelbiocare,
GStenborg, Sweden) also led to a
surface roughness of less than 0.2 pm
(0.13 pin and 0.11 Inn, respectively)
( Q u i r y n e n et al., 1996). Ceka
(Alphadent, La C h a u x - d e fonds,
Switzerland) and Prozyr abutments
(FBC Int. NV, Dessel, Belgium) even
had Ra values below 0.1 llm (0.05 pin
and 0.06 llm, respectively) (Bollen
et al., 1996) (Table 2).
Considering t h e t r e a t m e n t of
artifical a b u t m e n t s , special care
should be taken when titanium instru-
ments (Speelman et al., 1992), air
Lekness and Lie, 1991 Scaling 1.54 ± 0.29 powder abrasive systems (Eliades
+ Polishing (pumice) 126 ± 0.19 et al., 1991) or prophylactic agents,
+ Polishing (chalk) 1.10 ± 0.22 such as fluoride gels or gels contain-
Scaling 1.68 ± 0.40 ing hydrofluoric acid (PrSbster et al.,
+ Polishing (air powder) 1.41 ± 0.32 1992), are used. These products lead
+ Polishing (chalk) 1.19 ± 0.26 to a t r e m e n d o u s i n c r e a s e in the
initial s u r f a c e r o u g h n e s s of t h e
abutments.
Lutz et aL, 1995 0.03 Polishing(CCS 40) (Dentin) 0.03* Microbiology. When roughened
Polishing (Ddtartrine Z) 0.08" (Ra = 0.81 pro) abutments were com-
Polishing (Zircate) 0.11" pared to smooth ones (Ra = 0.35 pin)
Polishing (Cleanic) 0.15" (Quirynen et al., 1993), it was shown
Polishing (CCS 250) 0.23* that the rough abutments harbored 20
Polishing (Nupro Coarse) 0 25* times more bacteria subgingivally,
Polishing (pumice) 0.31" with a dearly higher proportion of
0.03 Polishing(CCS 40) (Enamel) 0.03* spirochetes and motile organisms,
Polishing (Cleanic) 0.03* which can be considered pathogenic
Polishing (Ddtartrine Z) 0.05*
(Theilade et al., 1966). w h e n the Ra
Polishing (COS 250) 0.06'
Polishing (Nupro Coarse) 0.06* value was decreased below 0.2 pm, no
Polishing (Zircate) 0,08* significant further changes in the
Polishing (pumice) 0.16" total amount nor in the pathogenicity
of the adhering-bacteria could be
detected (Quirynenetal., 1996; Bollen
et al., 1996). Therefore, it was stated
Artificial abutment on top of implants. The R, values of that 0.2 Inn is the threshold surface roughness below which
abutments in different studies are shown in Table 2. There no impact on the bacterial retention could be expected.
is a highly significant similarity between oral implants and Amalgam. The mean roughness values, before and
teeth, indicated by the presence of crevicular fluid, the after different treatment modalities, are depicted in
similarity in pocket epithelium or a comparable reaction Table 3.
toward plaque (Aspe et al., 1989; Sanz et al., 1991). When different brands of amalgam were compared,
Recently, Adonogianaki et al. (1995) even proved that the Smales (1981) found that the amalgams had a two-fold
260 Bollen et aL/Surface roughness of oral hard matenals: A review
the surface roughness of gold are
summarized in Table 4. Roulet
and R o u l e t - M e h r e n s (1982)
showed that polishing of gold with
different pastes did not have any
effect on the surface roughness.
Some pastes (Silperpolish and
Clean-polish) even smoothed the
gold surface from 0.04 tim to
a l m o s t 0.02 Bm. Nupro red
( J o h n s o n & Johnson, E a s t
Windsor, NJ, USA) and Nupro
green" (Johnson & J o h n s o n )
seemed to have an adverse effect
on the Ra value, but the roughness
still remained below 0.2 Bm.
Heath and Wilson (1976), in
contrast, showed that polishing of
initially smooth gold surfaces with
rubber cups, zirconium discs or a
prophylactic paste (Kemdent
Brand, Associated D e n t a l
Products, London, England, UK)
higher R, value than the threshold surface roughness, increased the surface roughness clearly above the
ranging from 0.43 ± 0.03 tim for Sybraloy (Sybron/Kerr, threshold R, value. Brushing had no effect on gold. The
Romulus, MI, USA) to 0.49 ± 0.04 tun for Indiloy (Shofu latter findings were confirmed by Johannsen et al. (1989;
Inc., Tokyo, Japan). 1992) who also r e p o r t e d t h a t b r u s h i n g gold with
Moreover, the finishing procedures were also found to toothpastes such as Clindomyn or Colgate did not have a
have a dramatic impact on the surface roughness. A study severe effect on the roughness. The application of scalers
by Roulet and Roulet-Mehrens (1982) showed that, when and other oral hygiene instruments on gold increased the
initially smooth amalgam (0.05 tim) was polished with R a value from 3 tim (use of curettes) to 20 tim (use of
different materials, the surface roughness remained cavitron) (Cutler et al., 1995) (Table 4).
below 0.2 tun. Some polishing pastes had no influence on No microbiological data were available.
the initial R, value: Fluor-O-Clean (Hawe Neos Dental, Resin composite. Many studies examined the surface
Gentilino, Switzerland), Zircate (L.D. Caulk Co., Dentsply roughness of resin composites (Table 5). When different
Int., Milford, DE, USA), Superpolish (Hawe Neos Dental) brands were studied in vitro, Willems et al. (1992) found
and Cleanpolish (Hawe Neos Dental). By contrast, that almost 30% of the 60 composites tested had an Ra
polishing of smooth amalgam (0.16 pm) with Clinomyn value below the threshold of 0.2 Bm after mechanical
and Colgate (Proctor and Gamble) toothpastes, resulted in polishing. Heliosit (Vivadent, Schaan, Liechtenstein),
a two- to three-fold increase in the surface roughness (up Certain (Johnson & Johnson) and Heliomolar (Vivadent)
to 0.56 Inn) (Johannsen et al., 1989). When initially rough were the smoothest (0.07 tim, 0.08 pm and 0.09 tim,
amalgam (R, = approximately 4.5 tim) was treated with respectively), whereas Estilux Posterior CVS (Kulzer
consecutive polishing and finishing procedures, a ten-fold Friedrichsdorf, Germany), Opalux (GC Int., Tokyo, Japan)
reduction in the surface roughness could be detected (Eide and Litefil A (Shofu Inc.) were initially the roughest
and Tveit, 1987). Davidson (1979) showed that polishing (1.48 tim, 1.50 Inn and 1.56 Inn, respectively). Smales
of amalgam with pumice and SnO 2 resulted in the (1981) detected higher in vitro values for Isopast (Vivadent)
smoothest surfaces and that the threshold roughness of (0.93 + 0.12 Inn) and Concise (3M Dental Products, St. Paul,
0.2 Inn could be reached. Other studies confirmed these MN, USA) (2.09 ± 0.08 Inn), whereas Willems et al. (19923
findings. The use of white stone (AD Trade Dist. Ltd., scored them as 0.13 tim and 1.44 tun, respectively.
London, England, UK) or Nupro-paste (Nupro-gold, Janar Possible explanations for these differences could be the
Co., Grand Rapids, MI, USA), polishing with 240-grit discs finishing procedures of the composites in the different
and tiny strips, are procedures that enormously increase studies and the different equipment used to perform the
the surface roughness (Table 3). surface roughness measurements. When finishing and
No microbiological data were available. However, one polishing procedures are considered, it is concluded that
can state that the amounts of copper (for T2-amalgam: from compressing a composite against a matrix creates a very
1.4 to 5.3%; for non T2-amalgam:from 8.6 to 29.7%) (Vrijhoef smooth surface. All studies showed R, values far below
et al., 1980) and mercury in the different amalgams have the threshold level of 0.2 tim, ranging from 0.03 tim
an antibacterial function. Nourallahi and Meryon (1989) (Restodent (Lee Pharmaceutics) (Davidson,. 1979) up to
showed t h a t when released from dental restorative 0.2 Inn (Adaptic (Johnson & Johnson, New Brunswick, NJ,
materials, copper and mercury, have an antibacterial USA) (Weitman and Eames, 1975). However, when
activity on bacterial plaque. conventional and microfilled composites were polished with
Gold. T h e results of different treatment modalities on different prophylactic pastes, an increase in surface
Dental Materials~July 1997 261
Heath and Wilson, 1976 Brushing with dentifrice 0.30*
Polishing (pumice) 0.50*
Polishing (rubber cup) 0.50*
Polishing (prophylactic paste) 0.60*
Polishing (Zr Silicat discs) 1.00"
+ Finishing bur 1.40"
+ Polishing (pumice) 0.40*
+ Polishing (chalk) 0.35*
+ Polishing (Sn02) 0.30*
4.20 Green stone 1.70"
+ Finishing bur 1.40*
+ Coarse polisher 0.50*
+ Fine polisher 0.20*
+ Polishing (pumice) 0.50*
+ Polishing (chalk) 0.40*
+ Polishing (SnO~) 0.40*
5.00 Medium sandpaper 1.40"
+ Fine sandpaper 0.80*
Polishing (fine white disc) 0.40*
+ Polishing (pumice) 0.45*
+ Polishing (chalk) ~ 0.40*
+ Polishin~L(Sp..O~" ~ 0.40*
~ ~ ~ ~ ~ ~ ~ : , ~ i ~ ; £ m ~ ; i ; 2 ~ ~ ~ ~
. ~ :. :.:~" .::. • .,~i~..~:~:~?~:,:~::~i~i~ . . ~ iii~ ~
roughness was detected, even up to 4 times (Roulet and decreased the surface roughness below the threshold R,
Roulet-Mehrens, 1982). Chung (1994) saw the opposite (0.18 pm and 0.14 Inn, respectively).
result: polishing Herculite (Kerr Mfg. Co., Romulus, MI, In studies where, unfortunately,no initial roughness was
USA) (0.35 ± 0.07 pm) and Heliomolar (0.52 ± 0.17 llm) mentioned, it can be concluded that only the polishing of
262 Bollen et aL/Surface roughness of oral hard materials: A review
between surface roughness and plaque forma-
tion and retention might be explained by the
high R a v a l u e s of t h e e x a m i n e d surfaces
Heath and Wilson, 1976 0.08 Brushing 0.07* (ranging from 0.8 to 1.4 l~n).
Polishing (prophylactic paste) 0.30* The cytotoxicity of monomers can also have
Polishing (rubber cup) 0.50* an antibacterial effect on the flora on this
material.
Polishing (Zr discs) 0.50*
Finishing (white stone) 0.90* Acrylic resin. The results of the treatment
of acrylic resin are not clear-cut (Table 6).
Busscher et al. (1984) stated that polishing this
material leads to a surface roughness below
the threshold R,, depending on the polishing
grit (from 0.03 lxm to 0.75 ]lm). Most other
studies found an increase in the roughness
after polishing procedures: Loney et al. (1994)
recently showed that treatment of resin with
different procedures leads to a two- to five-fold
Roulet and 0.04 Polishing(Superpolish) 0.02' increase of the surface roughness. Others
Roulet-Mehrens, 1982 Polishing (Cleanpolish) 0.03*
found a 10-fold increase when No. 400 emery
Polishing (Fluor-O-Clean) 0.03* paper was used (Verran et al., 1991). Similar
Polishing (Prophyprep) 0.03*
to composites, c o m p r e s s i n g acrylic resin
Polishing (Zircate) 0.03*
against glass results in a very low R, value
Polishing (Coral II) 0.04*
(0.10 ~m; Heath and Wilson, 1976) (Table 6).
Polishing (Nupro gold) 0.04* Microbiology. Verran et al. (1991) showed
Polishing (Colgate fluor prophylax paste) 0.05*
t h a t C a n d i d a a l b i c a n s cells a d h e r e d in
Polishing (Nupro green) 0.06" significantly higher numbers to rough acrylic
Polishing (Nupro red) 0.08* than to smooth acrylic, but maximal adhesion
was achieved on acrylic surfaces roughened
with medium grit size emery paper (R~ from
0.01 to 1.20 p~n).
The roughness of a denture-fitting surface
can help to d e t e r m i n e its colonization by
different microorganisms. Repeated aggressive
brushing of dentures with abrasive cleansers
will scratch even smooth denture surfaces
(Anon, 1983; De N a v a r r e , 1975). T h e s e
findings were confirmed by Yamamuchi et al.
(1990), who showed that Streptococcus sanguis,
Bacteroides gingivalis C-101 and Candida
albicans adhered in greater amounts to the
roughest surfaces in comparison to the smooth
surfaces.
Glass ionomers and compomers. Only three
studies concerning glass ionomer and
compomer cements could be found (Table 7).
Recently, Gladys et al. (1997) summarized data
on the surface roughness of these materials.
microfilled composites with alumina discs or with rubber Hotta et al. (1995) showed that only compressing Fuji~
wheels (O'Brien et al., 1984), the polishing of Isomolar with Ionomer II (GC Int.) against glass resulted in an, Rh ~ 0.2
diamond paste or Sof-lex discs (3M Dental Products) (Van llm. All the other manipulations of these cements, such as
dijken and Ruyter, 1987), and the polishing of Herculite polishing and glazing, showed higher surface roughness
with Sof-lex discs (Tjan and Chan, 1989) resulted in values, even up to 1.5 1Jm (polishing of Chelonfil (ESPE
s u r f a c e r o u g h n e s s b e l o w 0.2 l~m. All t h e o t h e r Fabrik, Seefeld, Germany) (Table 7).
manipulations led to a value above this threshold surface No microbiological data were available. The fluoride
roughness (Table 5). included in these materials resulted in antibacterial
Microbiology. Only the study by Skjorland et al. (1982) activity, although less i m p o r t a n t t h a n the activity of
mentioned some microbiological data: it was shown that copper, mercury and zinc in other dental restorative
bacteria a d h e r e d in similar amounts to the different materials (Nourollahi and Meryon, 1989; Cimasoni, 1972).
m a t e r i a l s a n d t h a t t h e r e did not a p p e a r to be a n y The fluoride release has a specific bactericidal effect on
predilection for the bacteria (Streptococcus sanguis ATCC mutans streptococci, but only for a relatively short period
10556) to accumulate in the voids or on the filler or matrix of time (Forss et al., 1991).
surface of the composite. The absence of a relationship Ceramics. The results for this material are summarized
Dental Materials~July 1997 263
w e r e s u b j e c t i v e l y e v a l u a t e d , it was
indicated that the integrity of the glazed
surface was altered in the form of deep
scratches on the ultrasonic-scaled surfaces
Weitman and Compressing (Mylar strip) 0.20* and numerous smaller scratches on the
Eames, 1975 Polishing (AIO) 0.71" hand-scaled porcelain. These scratches
Finishing (white Arkansas stone) 0.82* could not be detected with the profilometer.
Polishing (Zr paste) 1.01" Most finishing techniques were found to
Polishing (pumice) 1.44" render initially rough ceramic surfaces
smoother, below the threshold R~.
When ceramics with an initial R~ value
of nearly 3.0 ~ n were subjected to finish-
ing with Sof-lex discs, or carbide burs or
polishing with ET diamonds, with Flex-
discs or two striper diamonds, the rough-
ness decreased below 0.1 l~m. Ward et al.
(1995) showed that these findings were
true for different ceramic materials, such
Horton et a/., Compressing (Mylar strip) 0.04 ± 0.01 as Ceramco II (Ceramco Inc., Burlington,
1977 Polishing (discs) 0.58 + 0.11 NJ, USA),Vinytage (3M Dental Products),
Polishing (discs + Justi paste) 1.00 ± 0.10 Opal 58 (3M D e n t a l P r o d u c t s ) a n d
Polishing (discs + Precise paste) 1.02 ± 0.15 Duceram (Ducera Dental, GmBh, Rosbach,
Polishing (discs + 3M paste) 1.11 ± 0.12 Germany). Polishing with different
consecutive materials, such as tungsten,
Shofu points (Shofu Int.), diamond strips
(Premier Dental Co., Norristown, PA, USA)
or Sof-lex discs resulted in little surface
roughness, but not below the threshold
surface roughness (Whitehead et al., 1995)
(Table 8).
No microbiological data were available.
In summary, the roughness ofintraoral
hard surfaces had a major impact on the
retention of oral microorganisms. Supra-
as well as subgingivally, an increase in
surface roughness was found to result in a
faster colonization of the surfaces and a
faster maturation of the plaque, thereby
increasing the risk for caries and periodon-
tal inflammation. Therefore, the roughness
of all i n t r a o r a l h a r d s u r f a c e s should
approximate an Ra value of 0.2 ~m or
lower.
Whereas a lot of publications deal with
the supragingival aspect of surface rough-
ness, the technical difficulty of altering the
surface roughness of subgingival surfaces
without surgical intervention explains the
small number of publications concerning
this subject. Furthermore, the majority
of the articles are of relative importance
since only one roughness p a r a m e t e r is
mentioned (Ra, surface roughness). It
would have been more valid to compare
Rtm (mean of m a x i m u m peak to valley
heights of a profile over the assessment
length) or Sm (mean spacing between the
in Table 8. Lee et al. (1995) recently found that the use of profile peaks at the mean line) values since they give a
ultrasonic scalers or hand scalers had no influence on the better idea of the size of the irregularities on the surface.
roughness of initially smooth surfaces. Both treatment The Rtm value gives an idea of the depth of the defect,
modalities kept the roughness (< 0.14 ~m) below the whereas the Sm value describes the width of the defect.
threshold roughness, although when SEM photographs Bollen et al. (1996) and Quirynen et al. (1996) explained
264 Bollen et aL/Surface roughness of oral hard rnatedals: A review
b a c t e r i a a d h e r e to t h e s u r f a c e
parallel to the long axis of the
abutments. In addition, very few
publications could be found t h a t
Roulet and 0.28 Polishing (SuperpoUsh) (Conventional) 0.45* included microbiological data
Roulet-Mehrens, Polishing (Zircate) 0.85* related to the changes in surface
1982 Polishing (Cleanpolish) 0.70* roughness. From this point of view,
Polishing (Fluor-O-Clean) 0.80* a lot of research still must be done.
Polishing (Nupro gold) 0.85* Materials w i t h an initial low
Polishing (Coral II) 1.00" surface roughness should, there-
Polishing (Prophyprep) 1.00" fore, be selected as the first choice.
Polishing (Nupro green) 1.05" Amalgams with a low Ra value are
Polishing (Colgate fluoride prophylactic paste) 1.10' Sybraloy, ANA 68 or ANA 2000.
Polishing (Nupro red) 1.15" S m o o t h gold s u r f a c e s c a n be
0.05 Polishing (Superpolish) (Microfilled) 0.08* obtained with Herodor G, Sj5dings
Polishing (Zircate) 0.11" C, Primor and Try-cast soft. The
Polishing (Fluor-O-Clean) 0.12" l a r g e r e v i e w of c o m p o s i t e s by
Polishing (Nupro gold) 0.12' Willems et al. (1992) showed t h a t
Polishing (Cleanpolish) 0.14' several products are below the
Polishing (Coral II) 0.15" threshold level, such as Heliosit,
Polishing (Colgate fluoride prophylactic paste) 0.22* Certain and Heliomolar. From the
Polishing (Prophyprep) 0.22* glass ionomer c e m e n t s (Gladys
Polishing (Nupro green) 0.24* et al., 1997) only Fuji Ionomer
Polishing (Nupro red) 0.28* s e e m s to h a v e a n a c c e p t a b l e
surface roughness. Several acrylic
resins have an appropriate initial
Ra value: Ivoclar Sr Isosit PE,
Ivoclar SR Isosit N. Of the ceramic
m a t e r i a l s , o n l y V i t a V M K 68
O'Brien et aL, Polishing (rubber wheel) (Conventional) 0.30* porcelain had a surface roughness
1984 Polishing (Silicate disc) 0.32* reaching 0.2 pm.
Polishing (Alumina disc) 0.38* As for finishing procedures, the
Polishing (12-fluted bur) 1.50" b e s t w a y to a c h i e v e a s m o o t h
Polishing (rubber wheel) (Microfilled) 0.08* amalgam surface is to polish it with
Polishing (Alumina disc) 0.14" pumice or SnO=, whereas if one
Polishing (Silicate disc) 0.45* s t a r t s w i t h an i n i t i a l l y smooth
//
Polishing (12-fluted bur) 0.98* surface, most polishing pastes do
not increase the roughness very
much. Gold polishing can best be
performed with rouge. The optimal
w a y to m a k e r e s i n composites,
acrylic resin and glass ionomer
cements smooth is to compress
them against a polyester strip. Also
Vandijken and Polishing (SiC paper) (Isomolar) 0.04 + 0.0,' p o l i s h i n g w i t h a l u m i n a discs,
R~yter,1987 Polishing (Diamond paste 2.5 pm) 0.10 + 0.09 Sof-lex discs, rubber wheels or with
Polishing (Diamond paste 0.1 pm) 0.11 + 0.19 diamond pastes (from 7 to 0.1 pro)
Polishing (Diamond paste 1 pro) 0.11 + 0.05 can result in a smooth composite~
Polishing (Diamond paste 7 pm) 0.17 + 0.09 surface. Ceramics can preferably be
Polishing (Sof-lex, superfine) 0.19 + 0.06 finished w i t h Sof-lex or carbide
Polishing (Sof-lex, fine) 0.29 + 0.09 burs.
Brushing 0.30 + 0.18 ReceivedSeptember12, 1996/
Polishing (Sof-lex, medium) 0.39 + 0.13 AcceptedMarch14,1997
Polishing (Sof-lex, coarse) 1.04 + 0.3t Addresscorreslfondenceandreprintrequeststo:
(continued) CurdM.L.Bollen
Dept.of Periodontology,School of Dentistry
t h a t the Rtm value of all the artificial abutments they used OralPathologyand Maxillo-facialSurgery
was always less t h a n 1 pm, which means that vertically, CatholicUniversityofLeuven
bacteria which n o r m a l l y are several ~m in size are Capucijnenvoer7,B-3000Leuven,
protected against shear forces. The Sm values of the BEIL]IUM
abutments ranged from 26 to 6 gm, so only horizontally is Phone:++0032-16-332407
some shelter offered to the bacteria, but only if the Fax:++0032-16-332484
Dental Materials~July 1997 265
immunoglobulin G against
Porphyromonas gingivalis in
peri-implant crevicular fluid: A
comparison with gingival crevicu-
Vandijken and Polishing (SiC paper) (Concise 1985) 0.25 -,-0.06
lar fluid. Clin Oral Imp Res 6:14-
Ruyter,1987 Polishing (Sof-lex, superfine) 0.26 20.10
23.
Polishing (Sof-lex, fine) 0.35 +0.10
Anon P (1983). Denture cleansers and
Polishing (Sof-lex, medium) 0.48 20.16
fixatives. In:'~hich?" Consumers'
Brushing 1.05 ± 0.29
Association, London, Jan:29-33.
Polishing (Sof-lex, coarse) 1.11 ± 0.20
Aspe P, Ellen RP, Overall CM, Zarb
Polishing (Diamond paste 0.1 pro) 1.33 ± 0.33
GA(1989). Microbiota and crevicu-
Polishing (Diamond paste 2.5 pm) 1.41 + 0.20
lar fluid collagenase activity in the
Polishing (Diamond paste 7 pro) 1.78 + 0.30
osseointegrated dental implant
sulcus: A comparison of sites in
edentulous and partial edentulous
patients. J Periodontal Res 24:96-
105.
Bollen CML, Papaioannou W, Van
Eldere J, Schepers E, Quirynen M,
van Steenberghe D (1996). The
influence of a b u t m e n t surface
roughness on plaque accumulation
and peri-implant mucositis. Clin
Oral Imp Res 7:201-211.
Brecx M, Theilade J, AttstrSm R
(1983). An ultrastuctural quanti-
Tjan and Chan, Polishing (Luster paste) (Herculite) 0.11 20.02 tative study of the significance of
1989 Polishing (3M Sof-lex superfine) 0.11 ±0.03 microbial m a n i p u l a t i o n during
Polishing (3M Sof-lex fine) 0.16 .* 0.04 early d e n t a l p l a q u e growth.
Polishing (3M Sof-lex medium) 0.25 + 0.08 J Periodontal Res 18:177-186.
Polishing (white stone) 0.43 ± 0.15 Busscher HJ, Van Pelt AWJ, De Boer
Polishing (coarse) 0.84 + 0.19 P, De Jong HP, Arends J (1984).
Polishing (Luster paste) (P-30) 0.26 ± 0.03 The effect of surface roughening of
Polishing (3M Sof-lex superfine) 0.28 ± 0.03 polymers on m e a s u r e d contact
Polishing (3M Sof-lex fine) 0.32 ± 0.0 angles of liquids. Colloids Surfaces
Polishing (3M Sof-lex medium) 0.47 + 0.06 9:319-331.
Polishing (white stone) 0.70 ± 0.20 Chung K (1994). Effects of finishing
Polishing (coarse) 1.10 + 0.33 and polishing procedures on the
surface texture of resin composites.
Dent Mater 10:325-330.
Cimasoni G (1972). The inhibition of
enolase by fluoride in vitro. Caries
Willems et al., Heliosit 0.07" Res 6:93-102.
1992 Certain 0.08* Cooley RL, Lubow RM, Patrissi GA
Heliomolar 0.09* (1986). The effect of an air-powder
Estilux Posterior CVS 1.48" abrasive instrument on composite
Opalux 1.50" resin. J A m Dent Assoc 112:362-
Litefil A 1.56* 364.
Creaven PJ, Dennison JB,
Charbeneau GT (1980). Surface
roughness of two dental amalgams
after various polishing techniques.
J Prosthet Dent 43:289-297.
Cutler BJ, Goldstein GR, SimoneUi G
(1995). The effect of dental prophy-
laxis instruments on the surface
roughness of metals used for metal
ceramic crowns. J Prosthet Dent
73:219-222.
REFERENCES Davidson CL (1979). Enige aspecten van het afwerken
Adonogianaki E, Mooney J, Wennstrom JL, Lekholm U, van composieten. Belgisch Tijdschrift Tandheelkunde
K i n a n e DF (1995). A c u t e - p h a s e p r o t e i n s a n d 34:83-98.
266 Bollen et al./Surface roughness of oral hard materials: A review
Gladys S, Van
Meerbeek
B, B r a e m
M
Heath and Wilson, 1976 Compressing against glass 0.10"
Lambrechts
Compressing against mylar strip 0.30"
P, Vanherle
Polishing (rubber cup) 0.30*
G (1997).
Brushing 0.40*
Compara-
Polishing (prophylactic paste) 0.50*
t i v e
Polishing (pumice) 0.50*
physico-
Polishing (white stone) 1.00"
mechanical
Polishing (Zr discs) 1.00"
character-
~:--- ~i ization of
new hybrid
restorative
materials
w i t h con-
ventional
ii~' ~i~ glass-
ionomer
Johannnsen eta/., 1989 0.16 Polishing (Colgate) (Isosit PE) 0.14" and resin
Polishing (Clinomyn) 0.64* composite
0.16 Polishing (Colgate) (Isosit N) 0.44* restorative
Polishing (Clinomyn) 0.90* materials. J
Dent Res
76:883-
894.
Yamamuchi et aL, 1990 Polishing (No. 2000 emery paper + buff polishing) 0.09* Green E,
Polishing (No. 2000 emery paper + Perma cure polishing) 0.22* Ramfjord
Polishing (No. 2000 emery paper) 1.12" SP (1966).
Tooth
roughness
Loney et aL, 1994 1.20 Prolstc Wheel (+ polishing with SnO / + polishing with pumice) 3.3 + 0.7 / 2.3 + 0.2 / 1.4 + 0.2 a f t e r sub-
E-cutter (+ polishing with SnO / + polishing with pumice) 3.8+0.4/3.4+0.3/1.9±0.0 gingival
Moloplast St (+ polishing with SnO / + polishing with pumice) 4.0+0.4/2.6+0.211.4+0.2 rootplaning.
Moloplast C+S (+ polishing with SnO / + polishing with pumice) 4.2±0.1/1.9±0.1/1.7±0.0 J Perio-
Green (+ polishing with SnO / + polishing with pumice) 4.6 + 0.4 / 1.9 + 0.1 / 1.5 + 0.4 dontol
G)cutter (+ polishing with SnO / + polishing with pumice) 5.6 + 0.4/1.8 ± 0.4• 1.2 ± 0.1 37:396-
Moloplast cutter (+ polishing with SnO / + polishing with pumice) 5.7 ± 0.6/3.9 ± 0.3• 1.2 ± 0.1 399.
Sandpaper (+ polishing with SnO / + polishing with pumice) 7.2+0.6/2.3+0.2/1.4±0.1 Heath JR, Wil-
son HJ
(1976). Sur-
De Navarro MG (1975). Chemistry and manufacture of face roughness of restorations. Br Dent J 140:131-
cosmetics. Second ed., Volume III. Orlando: 137.
Continental Press. Horton CB, Paulus HM, Pelleu GB, Rudolph J J (1977). An
De Wet FA (1980). Dental glazes - Surface roughness and evaluation of commercial p a s t e s for finishing
plaque accumulation. Quintessence Int 9:127-136. composite resin surfaces. J Prosthet Dent 37:674-
Eide R, Tveit AB (1987). A comparison of different 679. '~
techniques for finishing and polishing amalgam. Acta Hotta H, Hirukawa H, Aono M (1995). The effect of glaze
Odontol S c a n d 45:147-151. on restorative glass-ionomer cements: Evaluation of
Eliades GC, Tzoutzas JG, Vougiouklakis GJ (1991). environmental durability in lactic acid solution. J Oral
Surface alterations on dental restorative materials Rehabil 22:685-689.
subjected to an air-powder abrasive instrument. Johannsen G, Redmalrn G, Ryd6n H (1989). Surface
J Prosthet Dent 65:27-33. changes on dental materials, i. The influence of two
Feh~r A, Miirmann WH (1995). Die Ausarbeitung van different dentifrices on surface roughness measured by
Keramikrestaurationen mit superfeinen Diamant- laser retie]don and profilometric techniques. Swed Dent
instrumenten. SchweizMonatsschrZahnmed 105:474- J 13:267-276.
479. Johannsen G, Redmalm G, Ryd6n H (1992). Surface
Forss H, Jokinen J, Spets-Happonen S, Seppii L, Luoma H changes on dental materials. II. The influence of two
(1991). Fluoride and mutans streptococci in plaque different dentifrices on surface roughness measured by
grown on glass ionomer and composite. Caries Res laser retie]don and profilometric techniques. S w e d Dent
25:454-458. J 16:13-20.
Dental Materi~s/July 1997 267
commercially pure titanium
abutments. Int J Oral & MaxiUofac
Implants 9:71-76.
Quirynen M, Bollen CML, Papaioannou
W, Van Eldere J, van Steenberghe D
(1996). The influence of t i t a n i u m
a b u t m e n t s surface r o u g h n e s s on
plaque accumulation and gingivitis.
Short-term observations. Int J Oral
& Maxillofac Implants 11:169-178.
Quirynen M, Marechal M, Busscher HJ,
W e e r k a m p AH, D a r i u s PL, v a n
Steenberghe D (1990). The influence
of surface free energy and surface
roughness on early plaque formation.
J Clin Periodontol 17:138-144.
Gladys et aL, 1996 Polishing procedures Ionosit 0.09 ± 0.01 Quirynen M, Van der Mei HC, Bollen
Ketac-Fil 0.29±0.04 CML, S c h o t t e A, M a r e c h a l M,
HIFI Master Palette 0.52 ± 0.10 Doornbusch GI, Naert I, Busscher HJ,
Photac-Fil 0.84 ± 0.39 van Steenberghe D (1993). An in vivo
Abrasion Ketac-Fil 1.07 ± 0.14 study of the influence of the surface
Ionosit 1.39 ± 0.071 roughness of implants on the microbi-
HIFI Master Palette 1.80± 0.18 : ology of supra-and subgingival plaque.
Photac-Fil 3.10 ± 0.40 JDent Res 72:1304-1309.
Lee SY, Lai YL, Morgano SM (1995). Effects of ultrasonic Rosenberg RM, Ash MM (1974). The effect of root
scaling and periodontal curettage on surface roughness r o u g h n e s s on plaque a c c u m u l a t i o n and gingival
of porcelain. JProsthet Dent 73:227-232. inflammation. J Periodontol 45:146-150.
Lekness KN, Lie T (1991). Influence of polishing Roulet JF, Roulet-Mehrens TK (1982). The surface
procedures on sonic scaling root surface roughness. roughness of restorative materials and dental tissues
J Periodontol 62:659-662. after polishing with prophylaxis and polishing pastes.
Loney RW, Moulding MB, Hacker CH (1994). Finishing J Periodontol 53:257-266.
and polishing of a Poly(fluoroalkoxyphosphazene) Sanz M, Alandez J, Lazaro P, Calvo JL, Quirynen M, van
resilient denture liner. Int J Prosthodont 7:362-367. Steenberghe D (1991). Histepathologic characteristics
Lutz F, Imfeld T, Schfipbach P (1995). Prophylaxepasten- of peri-implant soft tissues in Brtmemark implants with
Das neue Abrasiv Perlit im Vergleich zu koventionellen 2 distinct clinical and radiological patterns. Clin Oral
PutzkSrpern. Schweiz Monatsschrift Zahnmedizin Imp Res 2:128-134.
105:30-39. S k j o r l a n d KK, H e n s t e n - P e t t e r s e n A, O r s t a v i k D,
McGuckin RS, Babin JF, Meyer BJ (1992). Alterations in SSderholm KJ (1982). Tooth coloured dental restorative
h u m a n enamel surface morphology following vital materials: Porosities and surface topography in relation
bleaching. J Prosthet Dent 68:754-760. to bacterial adhesion. Acta Odontol Scand 40:113-120.
Nourollahi M, Meryon SD (1989). The antibacterial Slop D, Arends J (1987). Surface roughening of h u m a n
properties of four elements released from dental enamel a_P~ertoothbrushing. JBiologie Buc 15:159-162.
restorative materials. Int Endod J 22:9-16. Smales RJ (1981). Plaque growth on dental restorative
Nyvad B, Fejerskov O (1987). S c a n n i n g electron materials. JDent 9:133-140.
microscopy of early microbial colonisation of h u m a n Smales RJ, Creaven PJ (1979). Evaluation of clinical
enamel and root surface in vivo. Scand J Dent Res m e t h o d s for assessing t h e surface r o u g h n e s s of
95:287-296. restorations. J Prosthet Dent 42:45-52.
O'Brien WJ, Johnston WM, Fanian F, Lambert S (1984). Smith BA, Shanbour GS, Caffesse RG, Morrison EC,
The surface roughness and gloss ofcemposites. JDent Dennisson JD (1986). In vitro polishing effectiveness of
Res 63:685-688. interdental aids on root surfaces. J Clin Periodontol
P r a t t e n DH, J o h n s o n GH (1988). An evaluation of 13:597~603.
finishing instruments for an anterior and posterior Speelman JA, Collaert B, Klinge B (1992). Evaluation of
composite. J Prosthet Dent 60:154-158. different methods to clean titanium abutments. Clin
PrSbster L, LinW, Hfitteman H (1992). Effect of fluoride Oral Imp Res 3:120-127.
prophylactic agents on titanium surfaces. Int J Oral & Theilade J, Wright WH, J e n s e n SB, Lo~ H (1966).
Maxillofac Implants 7:390-394. Experimental gingivitis in man. II. A longitudinal
Quirynen M, Bollen CML (1995). The influence of surface clinical and bacteriological investigation. JPeriodontal
roughness and surface-free energy on supra- and Res 1:1-13.
subgingival plaque formation in man. A review of the Tjan AHL, Chan CA (1989). The polishability of posterior
literature. J Clin Periodontol 22:1-14. composites. J Prosthet Dent 61:138-146.
Quirynen M, BoUen CML, Willems G, van Steenberghe D Vandijken JWV, Ruyter IE (1987). Surface characteristics
(1994). Comparison of surface characteristics of six of p o s t e r i o r c o m p o s i t e s after p o l i s h i n g a n d
268 Bollen et al./Sufface roughness of oral hard materials: A review
Von Mierau HD,
Wfistefeld L, Holler-
Hfibsch M, Spindler T,
H e r i n g B (1982).
Fehdr and M6rmann, 3.30 Finishing (Sof-lex extra fine) 0.03* Rauhigkeit-
1995 Finishing (Sof-lex fine) 0.04* s u n t e r s u c h u n g e n an
Finishing (Sof-lex medium) 0.05* vestibul~iren und
Machine finishing 0.10" lingualen Zahnober-
Finishing (Sof-lex rough) 0.15" fl~ichen. Deutsches
Finishing (proxoshape 8 pm) 0.20* zahndirztliches
Finishing (proxoshape 4 IJm) 0.30* Zeitschrift 37:176-180.
Finishing (proxoshape 15 pm) 0.40* Vrijhoef MMA,
Finishing (4 pm diamond) 0.50* Vermeersch AG,
Finishing (8 pm diamond) 0.80* S p a n a u f AJ (1980).
Finishing (15 IJm diamond) 1.30" Dental Amalgam.
Chicago: Quintessence
Publishing Co., Inc. 54-
66.
Ward et aL, 1995 3.18 Polishing (Flexi-disc system) (Ceramco II) 0.10 ± 0.04 Ward MT, Tate WH,
Polishing (Two striper diamonds) 0.14 + 0.04 Powers J M (1995).
+ fluted carbide bur 0.06 ± 0.02 Surface roughness of
Polishing (ET diamonds) O.t 9 ± 0.04 opalescent porcelains
+ finishing with carbide bur 0.07 ± 0.02 after polishing. Oper
Over-glazing 0.39 ± 0.11 Dent 20:106-110.
Polishing (Porcelain laminate polishing) 0.44 ± 0.10 Weitman RT, Eames WB
Self-glazing 0.52 + 0.10 (1975). Plaque accu-
Enhance finishing 0.70 + O.13 mulation on composite
Intraoral porcelain polishing 0.79 ± 0.12 surfaces after various
2.96 Polishing (Flexi-disc system) (Vinytage / Opal 58) 0.09 ± 0.05 finishing procedures. J
Polishing (Two striper diamonds) 0.10 ± 0.04 A m DentAssoc 91:101-
+ fluted carbide bur 0.05 ± 0.02 106.
Polishing (ET diamonds) 0.14 ± 0.05 Whitehead SA, Shearer
+ finishing with carbide bur 0.07 ± 0.02 AC, Watts DC, Wilson
Polishing (Porcelain laminate polishing) 0.37 ± 0.08 NHF (1995). Compari-
Self-glazing 0.43 ± 0.08 son of m e t h o d s for
Over-glazing 0.44 ± 0.09 m e a s u r i n g surface
Enhance finishing 0.60 + O.14 roughness of ceramic.
Intraoral porcelain polishing 0.77 ± 0.16 J Oral Rehabi122:421-
2.60 Polishing (ET diamonds) (Duceram) 0.05 ± 0.02 427.
+ finishing with carbide bur 0.05 + 0.02 Willems G, Lambrechts P,
Enhance finishing 0.06 + O.10 Braem M, Vuylsteke-
Polishing (Flexi-disc system) 0.06 + 0.03 Wauters M, Vanherle G
Polishing (Two striper diamonds) 0.11 ± 0.04 (1991). The surface
+ fluted carbide bur 0.07 ± 0.03 roughness of enamel-
Over-glazing 0.37 -~ 0.10 to-enamel contact
Polishing (Porcelain laminate polishing) 0.38 ± 0.11 areas compared with
Self-glazing 0.44 ± 0.09 the intrinsic roughness
Intra-oral Porcelain polishing 0.78 + 0.10 of dental resin compos-
ites. J Dent Res
70:1299-1305.
Willems G, Lambrechts P,
B r a e m M, Celis JP,
Vanherle G (1992). A
classification of dental
composites according
to their morphological
and mechanical char-
toothbrushing. Acta Odontol Scand 45:337-346. acteristics. Dent Mater 8:310-319.
Verran J, Lees G, Shakespeare AP (1991). The effect of Yamamuchi M, Yamamoto K, Wakabayashi M, Kawano J
surface roughness on the adhesion ofCandida albicans (1990). In vitro adherence of micro-organisms to
to acrylic. Biofouling 3:183-192. denture base resin with different surface texture. Dent
Mater J 9:19-24.
Dental Materials/July 1997 269
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Production Options For Psilocybin Making of The MagicDocument7 pagesProduction Options For Psilocybin Making of The MagicYingjie ZhouNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Dna WorksheetDocument8 pagesDna WorksheetLuis100% (6)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Bucket ListDocument10 pagesBucket ListJacob GilbrethNo ratings yet
- MindmapDocument1 pageMindmapSwapnil Tak25% (4)
- Build Your Own HypertrophyDocument33 pagesBuild Your Own Hypertrophyaeu3csd bdvt100% (1)
- Cephalometrics For You and Me SteinerDocument27 pagesCephalometrics For You and Me SteinerAndrea Duran Juarez67% (6)
- Iso 16140 1 2016Document11 pagesIso 16140 1 2016Ha Do Thi ThuNo ratings yet
- Community HealthDocument52 pagesCommunity Healthshangsyndrome100% (2)
- ALIAS Course FlyerDocument2 pagesALIAS Course FlyerMartin AdriazolaNo ratings yet
- Milleding1999 PDFDocument9 pagesMilleding1999 PDFMartin AdriazolaNo ratings yet
- Piis0109564104000429 PDFDocument8 pagesPiis0109564104000429 PDFMartin AdriazolaNo ratings yet
- Surface Free Energy Dental MaterialsDocument10 pagesSurface Free Energy Dental MaterialsMartin AdriazolaNo ratings yet
- Powder and Liquid: Lens Reveals Hidden Beauty of A Ceramist's CraftsmanshipDocument9 pagesPowder and Liquid: Lens Reveals Hidden Beauty of A Ceramist's CraftsmanshipMartin AdriazolaNo ratings yet
- Ceramic DentistryDocument14 pagesCeramic DentistryMartin AdriazolaNo ratings yet
- Dental Materials SurfaceDocument6 pagesDental Materials SurfaceMartin AdriazolaNo ratings yet
- Fio Força CochraneDocument77 pagesFio Força CochraneMartin AdriazolaNo ratings yet
- AAPD Instructions For Authors: Pediatric DentistryDocument8 pagesAAPD Instructions For Authors: Pediatric DentistryMartin AdriazolaNo ratings yet
- Concepts of Surface TensionDocument31 pagesConcepts of Surface TensionMartin AdriazolaNo ratings yet
- Exercise-1: History, Variation, MendelismDocument41 pagesExercise-1: History, Variation, MendelismAhxhsNo ratings yet
- 2016 - Chuyên Thái NguyênDocument12 pages2016 - Chuyên Thái NguyênRoland VietnamNo ratings yet
- Full Download Book Medical Epigenetics PDFDocument41 pagesFull Download Book Medical Epigenetics PDFandrew.lindsey981100% (14)
- Ils ExamDocument36 pagesIls ExamzsifyounusNo ratings yet
- Tumor Monocyte Content Predicts ImmunochemotherapyDocument28 pagesTumor Monocyte Content Predicts ImmunochemotherapyDi V.No ratings yet
- L-29Metabolism of ChromoproteinsDocument22 pagesL-29Metabolism of ChromoproteinsAshar AhmadNo ratings yet
- U080323E Quirk of Evolution ReportDocument5 pagesU080323E Quirk of Evolution ReportJegendren TanapalNo ratings yet
- Preview Guidelines For Vegetation Management AASHTODocument13 pagesPreview Guidelines For Vegetation Management AASHTOBárbara CastroNo ratings yet
- Nanotechnology: Done By-Naveeshi Lawanya, Samadhee Kiriwandalage and Minduli MedagodaDocument13 pagesNanotechnology: Done By-Naveeshi Lawanya, Samadhee Kiriwandalage and Minduli Medagodanalinda medagodaNo ratings yet
- Basic Science Note Primary FourDocument16 pagesBasic Science Note Primary Foursulaiman mohammedNo ratings yet
- The Body Container A New Perspective On The Body EgoDocument21 pagesThe Body Container A New Perspective On The Body EgoClo Mn100% (1)
- Selander 1991 - On The Nomenclature and Classification of The MeloidaeDocument31 pagesSelander 1991 - On The Nomenclature and Classification of The MeloidaecristianNo ratings yet
- 3 Placenta Previa Nursing Care PlansDocument7 pages3 Placenta Previa Nursing Care PlansAjay RajpootNo ratings yet
- College of Medical Laboratory Science Our Lady of Fatima University-VelenzuelaDocument33 pagesCollege of Medical Laboratory Science Our Lady of Fatima University-VelenzuelaClaire GonoNo ratings yet
- Metode Antioksidan AEACDocument7 pagesMetode Antioksidan AEACFira KuswandariNo ratings yet
- Learning Activity Sheet: Agri-Fishery Arts 11 Agricultural Crops Production NC IIDocument12 pagesLearning Activity Sheet: Agri-Fishery Arts 11 Agricultural Crops Production NC IIKM Nicolas AgcaoiliNo ratings yet
- Production and Marketing Systems of Sheep and Goats in Alaba, Southern EthiopiaDocument174 pagesProduction and Marketing Systems of Sheep and Goats in Alaba, Southern EthiopiaYegnanewNo ratings yet
- Curriculum VitaeDocument3 pagesCurriculum Vitaeanon-866464No ratings yet
- Bio ProjectDocument13 pagesBio ProjectASAD RAHMANNo ratings yet
- Janir Ty Datukan Department of Physical Sciences College of Science Philippine Normal UniversityDocument32 pagesJanir Ty Datukan Department of Physical Sciences College of Science Philippine Normal UniversityCharles Dave Mendoza100% (4)
- Options To Improve The Action of PROTACs in Cancer Development of Controlled Delivery NanoparticlesDocument13 pagesOptions To Improve The Action of PROTACs in Cancer Development of Controlled Delivery NanoparticlesHan GaoNo ratings yet
- Dec Pages 65 67 - Compressed PDFDocument3 pagesDec Pages 65 67 - Compressed PDFDrVirendra Kumar TanwarNo ratings yet
- SMEs in Health ResearchDocument192 pagesSMEs in Health ResearchdmaproiectNo ratings yet