Professional Documents
Culture Documents
Sulfation and How To Prevent It
Uploaded by
وليد موسى0 ratings0% found this document useful (0 votes)
11 views3 pagesbattery maintenance
Original Title
Youssef 2004
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentbattery maintenance
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
11 views3 pagesSulfation and How To Prevent It
Uploaded by
وليد موسىbattery maintenance
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 3
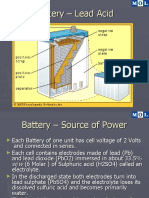
Sulfation and How to prevent it
Sulfation occurs when a lead acid battery is
deprived of a full charge. This is common with
starter batteries in cars driven in the city with load-
hungry accessories. A motor in idle or at low speed
cannot charge the battery sufficiently.
Electric wheelchairs have a similar problem in that
the users might not charge the battery long enough.
An eight-hour charge during the night when the
chair is free is not enough. Lead acid must
periodically be charged 14–16 hours to attain full
saturation. This may be the reason why wheelchair
batteries last only two years, whereas golf car
batteries deliver twice the service life. Longer
leisure time allows golf car batteries to get the fully
saturated charge.
Solar cells and wind turbines do not always provide
sufficient charge, and lead acid banks succumb to
sulfation. This happens in remote parts of the world
where villagers draw generous amounts of
electricity with insufficient renewable resources to
charge the batteries. The result is a short battery
life. Only a periodic fully saturated charge could
solve the problem, but without an electrical grid at
their disposal, this is almost impossible. An
alternative is using lithium-ion, a battery that is
forgiving to a partial charge, but this would cost
about six-times as much as lead acid.
What is sulfation? During use, small sulfate
crystals form, but these are normal and are not
harmful. During prolonged charge deprivation,
however, the amorphous lead sulfate converts to a
stable crystalline that deposits on the negative
plates. This leads to the development of large
crystals, which reduce the battery’s active material
that is responsible for high capacity and low
resistance. Sulfation also lowers charge acceptance.
Sulfation charging will take longer because of
elevated internal resistance.
There are two types of sulfation: reversible (or soft
sulfation), and permanent (or hard sulfation). If a
battery is serviced early, reversible sulfation can
often be corrected by applying an overcharge to a
fully charged battery in the form of a regulated
current of about 200mA. The battery terminal
voltage is allowed to rise to between 2.50 and
2.66V/cell (15 and 16V on a 12V mono block) for
about 24 hours. Increasing the battery temperature
to 50–60°C (122–140°F) further helps in dissolving
the crystals. Permanent sulfation sets in when the
battery has been in a low state-of-charge for weeks
or months. At this stage, no form of restoration is
possible.
There is a fine line between reversible and non-
reversible sulfation, and most batteries have a little
bit of both. Good results are achievable if the
sulfation is only a few weeks old; restoration
becomes more difficult the longer the battery is
allowed to stay in a low SoC. A sulfated battery
may improve marginally when applying a de-
sulfation service. A subtle indication of whether a
lead acid can be recovered is visible on the voltage
discharge curve. If a fully charged battery retains a
stable voltage profile on discharge, chances of
reactivation are better than if the voltage drops
rapidly with load.
Several companies offer anti-sulfation devices that
apply pulses to the battery terminals to prevent and
reverse sulfation. Such technologies tend to lower
sulfation on a healthy battery but they cannot
effectively reverse the condition once present.
Manufacturers offering these devices take the “one
size fits all” approach and the method is
unscientific. A random service of pulsing or blindly
applying an overcharge can harm the battery in
promoting grid corrosion. Technologies are being
developed that measure the level of sulfation and
apply a calculated overcharge to dissolve the
crystals. Chargers featuring this technique only
apply de-sulfation if sulfation is present and only
for the time needed.
You might also like
- Industrial Lead Acid Batteries: Types and Their SelectionDocument5 pagesIndustrial Lead Acid Batteries: Types and Their SelectionSellappan MuthusamyNo ratings yet
- Battery Handbook Jul2010 FINALDocument16 pagesBattery Handbook Jul2010 FINALBalu MNo ratings yet
- Marine Electrics Made Simple or How to Keep the Batteries ChargedFrom EverandMarine Electrics Made Simple or How to Keep the Batteries ChargedNo ratings yet
- 2011 Hyundai Service Electrical PartsDocument82 pages2011 Hyundai Service Electrical PartsJose QuirozNo ratings yet
- D6NDocument47 pagesD6NRodrigo Ricardo Véliz Meza100% (3)
- Lead Acid BatteriesDocument5 pagesLead Acid BatteriesRanjan KumarNo ratings yet
- Homebrew Lead Acid Battery DesulfatorDocument5 pagesHomebrew Lead Acid Battery Desulfatorrosslovelady100% (1)
- On Battery TestingDocument24 pagesOn Battery TestingMukund Chaudhary100% (1)
- Understanding Battery Maintenance 2018Document87 pagesUnderstanding Battery Maintenance 2018Iman RamangNo ratings yet
- Spare Parts ListDocument29 pagesSpare Parts ListRonaldNo ratings yet
- FAQ QuantaDocument11 pagesFAQ Quantarss56No ratings yet
- Komatsu PC 300-8 PDFDocument420 pagesKomatsu PC 300-8 PDFAbraham Huacasi100% (1)
- BatteryDocument10 pagesBatterymuhammad arifNo ratings yet
- Car Batteries LectureDocument11 pagesCar Batteries LectureArnel HimzonNo ratings yet
- All Types of Lead-Acid BatteriesDocument3 pagesAll Types of Lead-Acid BatteriesKamran Asghar100% (1)
- Email Sentences and Phrases in Different SituationsDocument11 pagesEmail Sentences and Phrases in Different SituationsOmer El-SayedNo ratings yet
- CTTestingTheory PDFDocument53 pagesCTTestingTheory PDFjengandxbNo ratings yet
- Owners Handbook - BUKH DV 24 RMEDocument24 pagesOwners Handbook - BUKH DV 24 RMELeonid Kolesnikov50% (2)
- Lead Acid Battery Desulfator GuideDocument5 pagesLead Acid Battery Desulfator GuidetopazeusNo ratings yet
- Lead Acid BatteriesDocument5 pagesLead Acid Batteriesz77iaNo ratings yet
- Basics of BatteryDocument6 pagesBasics of BatterysrisaitejaswiniNo ratings yet
- ABB Drives Function Blocks For Siemens PLCs Quick Start-Up Guide A A4Document40 pagesABB Drives Function Blocks For Siemens PLCs Quick Start-Up Guide A A4ElafanNo ratings yet
- Exide BtryDocument94 pagesExide BtryDivya ThakkerNo ratings yet
- High Voltage Cable PDFDocument2 pagesHigh Voltage Cable PDFNachoPérezCarrilloNo ratings yet
- Power-Sonic Battery GuideDocument20 pagesPower-Sonic Battery GuideGamal SalehNo ratings yet
- Hico SF Gas Circuit Breaker: Power Systems PUDocument6 pagesHico SF Gas Circuit Breaker: Power Systems PUSubir ChattopadhyayNo ratings yet
- Why lead-acid batteries sulfate and how to prevent itDocument2 pagesWhy lead-acid batteries sulfate and how to prevent itSergio Terceros Santa CruzNo ratings yet
- Lead-Acid Battery Sulfation and DesulfationDocument2 pagesLead-Acid Battery Sulfation and DesulfationFloyd PriceNo ratings yet
- 12.07 John Kim - Lead Acid Battery Charging Important Part of A Reliable Power Backup SystemDocument4 pages12.07 John Kim - Lead Acid Battery Charging Important Part of A Reliable Power Backup Systemgustavo RNo ratings yet
- BatriDocument10 pagesBatrithomas obiNo ratings yet
- BatteryDocument2 pagesBatteryaklatreaderNo ratings yet
- Service Department: Battery Basics How Do Lead Acid Batteries Work? Other Service ItemsDocument5 pagesService Department: Battery Basics How Do Lead Acid Batteries Work? Other Service Itemsrasheed313No ratings yet
- Battery BasicsDocument7 pagesBattery BasicsAnil kumarNo ratings yet
- VRLA Battery Lifetime Study - Factors Affecting Lead Acid Battery LifeDocument13 pagesVRLA Battery Lifetime Study - Factors Affecting Lead Acid Battery LifeRahul ShelkeNo ratings yet
- (Battery Types) : 1-Flooded Lead Acid BatteriesDocument3 pages(Battery Types) : 1-Flooded Lead Acid Batteriesahmed khaledNo ratings yet
- The Lead Acid BatteryDocument4 pagesThe Lead Acid BatterysathishNo ratings yet
- Service Department: Battery Basics How Do Lead Acid Batteries Work? Other Service ItemsDocument4 pagesService Department: Battery Basics How Do Lead Acid Batteries Work? Other Service ItemsONILEDA1970100% (1)
- Lead AcidDocument3 pagesLead AcidMiguel AyalaNo ratings yet
- Research Paper On Lead Acid BatteryDocument6 pagesResearch Paper On Lead Acid Batterygpnovecnd100% (1)
- MODULE 12. Batteries (Jogesh)Document26 pagesMODULE 12. Batteries (Jogesh)Sharapov Mechanic (Все просто)No ratings yet
- Common Causes of Premature Battery FailureDocument2 pagesCommon Causes of Premature Battery Failureavandetq15No ratings yet
- Shoprider Troubleshooting 2007Document36 pagesShoprider Troubleshooting 2007Glenn TrofatterNo ratings yet
- Automotive Battery PresentationDocument26 pagesAutomotive Battery PresentationJay De Lumen NacidoNo ratings yet
- Harging EAD CID: Learn How To Optimize Charging Conditions To Extend Service LifeDocument4 pagesHarging EAD CID: Learn How To Optimize Charging Conditions To Extend Service LifebigsloNo ratings yet
- Pulse Tech Revolutionizes Battery LifeDocument3 pagesPulse Tech Revolutionizes Battery LifeJaideep PoddarNo ratings yet
- Lead Acid BatteryDocument17 pagesLead Acid Batterymuksadur rahmanNo ratings yet
- The Battery: SubjectDocument36 pagesThe Battery: SubjectporkfaceNo ratings yet
- Automotive Electrical and Electronics EngineeringDocument5 pagesAutomotive Electrical and Electronics EngineeringKarthik SwaminathanNo ratings yet
- Mtekpro Proposal For Tata Motors On A/C of DTC, Delhi For Battery Testing in Delhi RegionDocument24 pagesMtekpro Proposal For Tata Motors On A/C of DTC, Delhi For Battery Testing in Delhi RegionAmaresh NayakNo ratings yet
- Battery-Guide Glo EngDocument21 pagesBattery-Guide Glo EngMd Rodi BidinNo ratings yet
- 10 How Lead Acid Batteries WorkDocument10 pages10 How Lead Acid Batteries Workfalejandro1971No ratings yet
- Battery Types Used For Auxiliary Power Supply in Substations and Power PlantsDocument8 pagesBattery Types Used For Auxiliary Power Supply in Substations and Power PlantsTigrilloNo ratings yet
- Basic Electronic ComponentsDocument31 pagesBasic Electronic ComponentsEngr. CasmirNo ratings yet
- Automatic Battery ChargerDocument16 pagesAutomatic Battery ChargerJULIET MOKWUGWONo ratings yet
- Lec 2Document12 pagesLec 2Joseph MagonduNo ratings yet
- Girma NewDocument41 pagesGirma NewEyayaw YemataNo ratings yet
- Assignment 01Document21 pagesAssignment 01Rexona KhanomNo ratings yet
- Different Types of Battery Used For Auxiliary Power Supply in Substations and Power PlantsDocument7 pagesDifferent Types of Battery Used For Auxiliary Power Supply in Substations and Power PlantsEngr. Abdullah100% (1)
- (GUIDE) Sealed Lead Acid BatteryDocument5 pages(GUIDE) Sealed Lead Acid BatteryDonnyKalsumajayaNo ratings yet
- Battery Starter AlternatorDocument8 pagesBattery Starter AlternatorIslam AlazbNo ratings yet
- Literature Review of Lead Acid BatteryDocument8 pagesLiterature Review of Lead Acid Batteryc5r3aep1100% (1)
- 3_ME467-Engine-ElectricDocument21 pages3_ME467-Engine-Electricnoman88407No ratings yet
- Comparison Between Li-Ion and Lead Acid BatteryDocument5 pagesComparison Between Li-Ion and Lead Acid BatterySAGAR RATHEENo ratings yet
- Digital Medium-Voltage Switchgear: Benefits, Offering and ReferencesDocument34 pagesDigital Medium-Voltage Switchgear: Benefits, Offering and Referencesوليد موسىNo ratings yet
- المحاضرة 3:إختبارات المفاتيح الحلقة RMUDocument34 pagesالمحاضرة 3:إختبارات المفاتيح الحلقة RMUوليد موسى100% (2)
- Comm Procedure For Genset Installlation in Govt Building HongkongDocument23 pagesComm Procedure For Genset Installlation in Govt Building Hongkongbhen08No ratings yet
- المحاضرة الرابعة:إختبارات محولات التوزيعDocument53 pagesالمحاضرة الرابعة:إختبارات محولات التوزيعوليد موسىNo ratings yet
- Testing and Commissioning of Medium Voltage Equipment VT TestsDocument26 pagesTesting and Commissioning of Medium Voltage Equipment VT Testsوليد موسىNo ratings yet
- Earthing Design Procedure & CalculationsDocument35 pagesEarthing Design Procedure & CalculationsAshwin NarayanNo ratings yet
- Installation and Service Instructions 12-40.5 KV - 630-3600 A - 16-50 KaDocument36 pagesInstallation and Service Instructions 12-40.5 KV - 630-3600 A - 16-50 Kaوليد موسىNo ratings yet
- Combined Fuzzy-Logic Wavelet-Based Fault Classification Technique For Power System RelayingDocument8 pagesCombined Fuzzy-Logic Wavelet-Based Fault Classification Technique For Power System Relayingوليد موسىNo ratings yet
- 47 PDFDocument5 pages47 PDFوليد موسىNo ratings yet
- High VoltageDocument17 pagesHigh Voltageوليد موسىNo ratings yet
- 180 Degree ConductionDocument27 pages180 Degree Conductionsathishsutharsan87No ratings yet
- High VoltageDocument17 pagesHigh Voltageوليد موسىNo ratings yet
- Helioscope Simulation 13233814 SummaryDocument3 pagesHelioscope Simulation 13233814 SummaryandisaputrawuNo ratings yet
- Eaton - 93PM - 30-200 - Datasheet - LOW 724Document2 pagesEaton - 93PM - 30-200 - Datasheet - LOW 724keyurNo ratings yet
- Sterner Infranor Polaris-12 Series Brochure 2006Document20 pagesSterner Infranor Polaris-12 Series Brochure 2006Alan MastersNo ratings yet
- 2,7,8, DP Aoh Condiciones TecnicasDocument3 pages2,7,8, DP Aoh Condiciones TecnicasFernando EncisoNo ratings yet
- Activities Undertaken For Development of Vendors Since April'2022Document6 pagesActivities Undertaken For Development of Vendors Since April'2022Ajay SinghNo ratings yet
- AS350Document57 pagesAS350Danna PerezNo ratings yet
- REN RC310xxA DST 20230531Document67 pagesREN RC310xxA DST 20230531杉澤 佑樹No ratings yet
- CV FormatDocument2 pagesCV FormatTehmas KamranNo ratings yet
- PVsyst Meralco Power House Merlin 105Wp + AbbDocument5 pagesPVsyst Meralco Power House Merlin 105Wp + AbbMARK ARQUE LACANARIANo ratings yet
- USB To Rs 32 ConvertorDocument11 pagesUSB To Rs 32 ConvertoryannickNo ratings yet
- CPE006 - Using Arduino: Technological Institute of The Philippines Manila Computer Engineering DepartmentDocument23 pagesCPE006 - Using Arduino: Technological Institute of The Philippines Manila Computer Engineering DepartmentPsalm San Juan TupazNo ratings yet
- Diesel - TDI - Axius - Gen - 2 - Option - Overview - Rev 5A - GeneralDocument8 pagesDiesel - TDI - Axius - Gen - 2 - Option - Overview - Rev 5A - GeneralJairPedroniNo ratings yet
- Instruction Manual FAA Types L-880 and L-881 Precision Approach Path Indicator Systems 88XA2X-X-XXPAPI DOCUMENT 2438Document73 pagesInstruction Manual FAA Types L-880 and L-881 Precision Approach Path Indicator Systems 88XA2X-X-XXPAPI DOCUMENT 2438Jose Manuel Restrepo Ruiz100% (2)
- Cone Ranger: Mobile Cone Crushing UnitsDocument2 pagesCone Ranger: Mobile Cone Crushing UnitsYousef AlipourNo ratings yet
- IR 2945 PartsListDocument2 pagesIR 2945 PartsListezeizabarrenaNo ratings yet
- STM 32Document8 pagesSTM 32Simone FontanaNo ratings yet
- Hägglunds CB motor data and dimensionsDocument1 pageHägglunds CB motor data and dimensionsThiago SilvaNo ratings yet
- Philips - Decorative LightingDocument9 pagesPhilips - Decorative LightingrumahsketchNo ratings yet
- Acer Extensa 5620-6419 Service Manual2Document157 pagesAcer Extensa 5620-6419 Service Manual2Patricia MalhaoNo ratings yet
- PTC Finned Resistor Heaters: Industrial Thermal ManagementDocument5 pagesPTC Finned Resistor Heaters: Industrial Thermal ManagementDanilNo ratings yet
- App 1238 PDFDocument3 pagesApp 1238 PDFIBRNo ratings yet
- Bahan 4Document9 pagesBahan 4wahyudhyNo ratings yet
- MODULAR DESIGN. INFINITE POSSIBILITIES. - Modern Furniture (PDFDrive)Document68 pagesMODULAR DESIGN. INFINITE POSSIBILITIES. - Modern Furniture (PDFDrive)Mee MeeNo ratings yet