Professional Documents
Culture Documents
Revision Questions
Uploaded by
DOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Revision Questions
Uploaded by
DCopyright:
Available Formats
Chapter 15
Revision questions
Page 308
1. (a) qualitative
(b) qualitative
(c) quantitative
(d) qualitative if only the presence of MSG was tested for or quantitative if the amount of
MSG was determined
2. (a) It would be necessary to qualitatively establish the presence of a possible source of the
oil waste before carrying out a more involved and expensive quantitative analysis.
(b) The polluting oil would have to be analysed to determine what compounds were
present in the mixture and in what quantities. The results of this analysis could then be
compared with the analysis of any residual waste oil found on a ship.
Page 310
3. Electrical conductivity is a measure of water’s ability to pass electrical flow (to carry
electricity). This ability is related to the total concentration of ions present in solution and is
not designed to detect the concentration of any particular ion.
Page 312
4. The strict protocols allow the production of accurate reproducible analytical results.
Page 317
5. The second sample was diluted by a factor of 10 (10 mL diluted to a total volume of 100
mL). Using the calibration curve, an absorbance reading of 0.790 gives a concentration ≈ 11
ppm; i.e. the diluted sample has a concentration ≈ 11 mg L–1 (1 ppm = 1 mg L–1). Therefore,
the undiluted sample has a concentration ≈ 10 × 11 = 110 mg L–1.
Page 320
6. (a) n(Ag2S) = 2/4 × n(Ag) = 2/4 × 1 = 0.5 mol
(b) n(H2S) = 2/4 × n(Ag) = 2/4 × 1 = 0.5 mol
(c) n(Ag2S) = n(H2S) = 3.5 mol
7. (a) n(CH4) = 1/2 × n(O2) = 1/2 × 1 = 0.5 mol
© John Wiley & Sons Australia, Ltd 1
(b) n(O2) = 2 × n(CH4) = 2 × 0.1 = 0.2 mol
(c) n(CO2) = n(CH4) = 0.1 mol
(d) n(H2O) = 2 × n(CH4) = 2 × 0.1 = 0.2 mol
(e) n(CO2) = 1/2 × n(O2) = 1/2 × 0.1 = 0.05 mol
(f) n(O2) = 2 × n(CH4) = 2 × 0.25 = 0.50 mol
(g) n(H2O) = n(O2) = 8 mol
Pages 321–2
8. CH4(g) + 2O2(g) CO2(g) + 2H2O(l)
n(CH4) = m/M = 2.8/16.0 = 0.175 mol
Therefore, n(H2O) = 2 × n(CH4) = 2 × 0.175 = 0.35 mol
and m(H2O) = n × M = 0.35 × 18.0 = 6.3 g (to 2 significant figures)
9. (a) n(O2) = m/M = 6.5/32.0 = 0.203 mol
n(H2O) = 4/5 × n(O2) = 4/5 × 0.203 = 0.163 mol
m(H2O) = n × M = 0.163 × 18.0 = 2.9 g (to 2 significant figures)
(b) n(C3H8) = m/M = 1.7/44.0 = 0.0386 mol
n(O2) = 5 × n(C3H8) = 5 × 0.0386 = 0.193 mol
m(O2) = n × M = 0.193 × 32.0 = 6.2 g (to 2 significant figures)
(c) n(C3H8) = 0.50 mol
n(CO2) = 3 × n(C3H8) = 3 × 0.50 = 1.5 mol
m(CO2) = n × M = 1.5 × 44.0 = 66 g (to 2 significant figures)
(d) n(CO2) = m/M = 5.92/44.0 = 0.135 mol
n(C3H8) = 1/3 × n(CO2) = 1/3 × 0.135 = 0.0448 mol
m(C3H8) = n × M = 0.0448 × 44.0 = 1.97 g
(e) n(C3H8) = m/M = 5.0 × 103/44.0 = 113.6 mol
n(CO2) = 3 × n(C3H8) = 3 × 113.6 = 340.9 mol
m(CO2) = n × M = 340.9 × 44.0 = 1.5 × 103 g = 15 kg (to 2 significant figures)
Page 339
10. n(KI) = m/M = 2.864/166.0 = 0.017 25 mol
n(PbI2) = 1/2 × n(KI) = 0.008 627 mol
m(PbI2) = n × M = 0.008 627 × 461.0 = 3.977 g
© John Wiley & Sons Australia, Ltd 2
11. (a) The barium carbonate is a solid.
(b) n(BaCO3) = m/M = 4.582/197.3 = 0.023 22 mol
n(BaCl2) = n(BaCO3) = 0.023 22 mol
m(BaCl2) = n × M = 0.023 22 × 208.3 = 4.837 g
Page 325
13. Zn(s) + H2SO4(aq) ZnSO4(aq) + H2(g)
n(H2SO4) = c × V = 0.250 × 0.400 = 0.100 mol
n(ZnSO4) = n(H2SO4) = 0.100 mol
m(ZnSO4) = n × M = 0.100 × 161.5 = 16.2 g (to 3 significant figures)
14. (a) n(Na2S) = c × V = 0.178 × 0.235 = 0.0418 mol
n(CdS) = n(Na2S) = 0.0418 mol
m(CdS) = n × M = 0.0418 × 144.5 = 6.04 g (to 3 significant figures)
(b) All of the Na2S will react.
15. Mg(s) + 2HCl(aq) MgCl2(aq) + H2(g)
(a) All the HCl had reacted — the reaction was complete.
(b) The mass of magnesium that reacted = 2.56 – 0.350 = 2.21 g.
n(Mg) that reacted = m/M = 2.21/24.3 = 0.0910 mol
n(HCl) that reacted = 2 × n(Mg) = 2 × 0.0910 = 0.182 mol
c(HCl) = n/V = 0.182/0.200 = 0.909 M
(c) Errors have a scientific basis as they relate to judgements made when measuring,
whereas mistakes relate to misjudgements, or human error, when measuring. Sources of error
in this experiment would be in weighing of the strip of magnesium and in measurement of the
volume of hydrochloric acid.
(d) Wear a safety coat, safety glasses and gloves, and work in a well-ventilated fume
cupboard or a well-ventilated laboratory.
Page 329
16.
8.81
n(CaO) =
56.1
= 0.157 mol
© John Wiley & Sons Australia, Ltd 3
m(CaCl2) = 0.157 × 111.1
= 17.5 g
17.5
Percentage purity =
18.0
= 96.9 %
Multiple choice questions
1. A 2. B 3. B 4. D 5. C 6. C 7. D 8. C 9. B 10. D 11. D 12. A 13. A
14. B 15. D
Review questions
1. (a) qualitative (b) qualitative (c) quantitative (d) quantitative
3. (a) A contaminant is an unwanted substance that makes water unsuitable for an intended
use.
(b) Water discharged from a sewage treatment plant would be unsuitable for use as
drinking water but it would be very good for irrigating market gardens where a variety of
fruit and vegetables are being grown.
(c) Not all contaminants cause problems. Sometimes creative thinking can find solutions to
perceived problems.
6. (a) Ba(NO3)2(aq) + Na2SO4(aq) 2NaNO3(aq) + BaSO4(s)
The precipitate is BaSO4.
(b) n(Ba(NO3)2) = m/M = 5.10/261.3 = 0.0195
n(BaSO4) = n(Ba(NO3)2) = 0.0195
m(BaSO4) = n × M = 0.0195 × 233.4 = 4.55 g (to 3 significant figures)
7. (a) n(N2) = 1/2 × n(NO) = 1/2 × 1.52 = 0.760 mol
(b) n(O2) = n(N2) = 0.760 mol
m(O2) = n × M = 0.760 × 32.0 = 24.3 g
8. (a) 2C(s) + O2(g) 2CO(g) assuming charcoal briquettes to be pure carbon, C
n(C) = m/M = 3.5/12.0 = 0.29 mol
n(O2) = 1/2 × n(C) = 0.15 mol (to 2 significant figures)
(b) C(s) + O2(g) CO2 (g)
n(C) = m/M = 3.5/12.0 = 0.29 mol
© John Wiley & Sons Australia, Ltd 4
n(O2) = n(C) = 0.29 mol
m(CO2) = n × M = 0.29 × 44.0 = 13 g (to 2 significant figures)
9. (a) 2Na(s) + Cl2(g) 2NaCl(s)
(b) n(Na) = m/M = 10.0/23.0 = 0.435
n(NaCl) = n(Na) = 0.435
m(NaCl) = n × M = 0.435 × 58.5 = 25.4 g
10. (a) n(C6H12O6) = m/M = 8.90/180.0 = 0.0494 mol
n(O2) = 6 × n(C6H12O6) = 6 × 0.0494 = 0.296 mol
m(O2) = n × M = 0.296 × 32.0 = 9.49 g
(b) n(C6H12O6) = m/M = 8.90/180.0 = 0.0494 mol
n(CO2) = 6 × n(C6H12O6) = 6 × 0.0494 = 0.296 mol
m(CO2) = n × M = 0.296 × 44.0 = 13.1 g
11. n(Ag) = m/M = 0.025/107.9 = 0.000 23 mol
n(Ag2S) = 1/2 × n(Ag) = 1/2 × 0.000 23 = 0.00012 mol
n(Ag2S) = n × M = 0.000 12 × 247.9 = 0.029 g (to 2 significant figures)
12. n(H2S2O7) = m/M = 5.00 × 103/178.2 = 28.1 mol
n(H2SO4) = 2 × n(H2S2O7) = 2 × 28.1 = 56.2 mol
m(H2SO4) = n × M = 56.2 × 98.1 = 5.51 × 103 g = 5.51 kg
13. (a) n(WO3) = m/M = 200/231.8 = 0.863 mol
n(W) = n(WO3) = 0.863 mol
m(W) = n × M = 0.863 × 183.8 = 159 g
(b) n(H2) = 3 × n(WO3) = 3 × 0.863 = 2.59
m(H2) = n × M = 2.59 × 2.0 = 5.2 g (to 2 significant figures)
14. (a) Cu(s) + 2AgNO3(aq) Cu(NO3)2(aq) + 2Ag(s)
(b) m(Cu) = 4.36 – 2.21 = 2.15 g
n(Cu) = m/M = 2.15/63.5 = 0.0339 mol
n(Ag) = 2 × n(Cu) = 2 × 0.0339 = 0.0678 mol
m(Ag) = n × M = 0.0678 × 107.9 = 7.31 g (to 3 significant figures)
15. Pb2+(aq) + SO42–(aq) PbSO4(s)
n(PbSO4) = m/M = 0.0806/303.3 = 0.000 266 mol
n(Pb2+) = n(PbSO4) = 0.000 266 mol
© John Wiley & Sons Australia, Ltd 5
m(Pb2+) = n × M = 0.000 266 × 207.2 = 0.0551 g
%Pb2+ in the pigment = (mass of Pb2+/mass of pigment) × 100
= (0.0551/1.50) × 100 = 3.67 %
16. n(Al) = m/M = 5.0/27.0 = 0.19 mol
n(Al2O3) = 2/4 × n(Al) = 2/4 × 0.19 = 0.095
m(Al2O3) = n × M = 0.095 × 102.0 = 9.7 g (to 2 significant figures)
Note that a different treatment of significant figures (where you keep all information
in your calculator until the final answer is achieved before rounding to the correct
number of significant figures) can produce a small variation in your answer. Both
answers are correct! For example:
n(Al) = m/M = 5.0/27.0 = 0.185 185 ... mol
n(Al2O3) = 1/2 × n(Al) = 1/2 × 0.185 185 = 0.092 592 5 mol
m(Al2O3) = n × M = 0.092 592 5 ... × 102.0 = 9.4 g (to 2 significant figures)
17. (a) n(LiOH) = m/M = 1.00 × 103/23.9 = 41.8 mol
n(CO2) = 1/2 × n(LiOH) = 1/2 × 41.8 = 20.9 mol
m(CO2) = n × M = 20.9 × 44.0 = 921 g = 0.921 kg
(b) 2NaOH(s) + CO2(g) Na2CO3(aq) + H2O(l)
(c) n(NaOH) = m/M = 1.00 × 103/40.0 = 25 mol
n(CO2) = 1/2 × n(NaOH) = 1/2 × 25 = 12.5 mol
m(CO2) = n × M = 12.5 × 44.0 = 550 g
(d) When planning space travel, there is a focus on minimising the mass of material that
needs to be lifted into space. Lithium hydroxide would be preferred because a lower mass of
lithium hydroxide would be needed to remove the same mass of carbon dioxide.
(e) 2OH–(s) + CO2(g) CO32–(aq) + H2O(l) for both reactions mentioned
18. (a) 1 tonne = 106 g. 100 tonnes of limestone would contain 100 × 83.5/100 = 83.5 tonnes
= 83.5 × 106 g of CaCO3.
n(CaCO3) = m/M = 83.5 × 106/100.1 = 8.34 × 105 mol
n(CaO) = n(CaCO3) = 8.34 × 105 mol
m(CaO) = n × M = 8.34 × 105 × 56.1 = 4.68 × 107 g = 46.8 tonnes
(b) The term ‘burning’ usually means the combustion of a substance with the oxygen in air.
19. n(Ca(OH)2) = m/M = 2.5/74.1 = 0.034 mol
n(HCl) = 2 × n(Ca(OH)2) = 2 × 0.034 = 0.068 mol
© John Wiley & Sons Australia, Ltd 6
Since c = n/V, then V = n/c.
V(HCl) = n(HCl)/c(HCl) = 0.068/1.3 = 0.052 L = 52 mL
20. n(AgNO3) = c × V = 0.100 × 0.250 = 0.0250 mol
n(Cu) = 1/2 × n(AgNO3) = 1/2 × 0.0250 = 0.0125 mol
m(Cu) = n × M = 0.0125 × 63.5 = 0.794 g
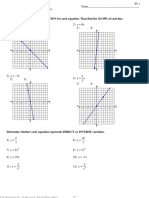
22. (a) zero
(b)
(c) concentration ≈ 2.2 ppm
23. (a)
© John Wiley & Sons Australia, Ltd 7
(b) concentration of sample 1 ≈ 41 mg L–1
concentration of sample 2 ≈ 5 mg L–1
24. (a)
(b) The level of mercury is ≈ 7.2 mg L–1.
(c) AAS is used specifically for the analysis of metal ions in solution.
25. (a) 16 ppm
(b) Concentration in the 20.0 mL sample = 16 ppm ≡ 16 mg L–1.
This will be the same as the concentration in the original 1000 mL solution.
Therefore, the mass of phosphorus (P) in the 1000 mL of detergent = 16 mg, and the mass of
phosphorus, P, in the 1.000 g sample of detergent = 16 mg.
16 mg = 0.016 g
%P by mass = (mass of P/mass of detergent) × 100
= (0.016/1.000) × 100
= 1.6 %
26. (a) AgNO3(aq) + NaCl(s) AgCl(s) + NaNO3(aq)
Ag+(aq) + Cl–(aq) AgCl(s)
(b) BaCl2(aq) + Na2SO4(aq) BaSO4(s) + 2NaCl(aq)
Ba2+(aq) + SO42–(aq) BaSO4(s)
(c) Pb(NO3)2(aq) + K2CrO4(aq) PbCrO4(s) + 2KNO3(aq)
Pb2+(aq) + CrO42–(aq) PbCrO4(s)
© John Wiley & Sons Australia, Ltd 8
(d) Na3PO4(aq) + 3AgNO3(aq) Ag3PO4(s) + 3NaNO3(aq)
PO43–(aq) + 3Ag+(aq) Ag3PO4(s)
(e) 2HCl(aq) + Pb(NO3)2(aq) PbCl2(s) + 2HNO3(aq)
2Cl–(aq) + Pb2+(aq) PbCl2(s)
27. (a) soluble (b) insoluble (c) insoluble (d) insoluble (e) soluble (f) soluble
28. (a) mass of water = 124.829 – 107.214 = 17.615 g
% water in the soil = (17.615/124.829) × 100 = 14.11%
(b) to ensure that all of the water has been removed
(c) There would be no way of ensuring that all of the water has been removed.
29. n(As2S3) = m/M = 0.353/246.1 = 0.00143 mol
As2S3(aq) 2As3+(aq) + 3S2–(aq)
n(As) = n(As3+) = 2 × As2S3 = 2 × 0.001 43 = 0.002 86 mol
and m(As) = n × M = 0.002 86 × 74.9 = 0.214 g
%As in the pesticide = (mass of As/mass of pesticide) × 100
= (0.214/2.15) × 100
= 9.95%
Note that a different treatment of significant figures (where you keep all information
in your calculator until the final answer is achieved before rounding to the correct
number of significant figures) can produce a small variation in your answer. Both
answers are correct! For example:
n(As2S3) = m/M = 0.353/246.1 = 0.001 434 37 ... mol
As2S3(aq) 2As3+(aq) + 3S2–(aq)
n(As) = n(As3+) = 2 × As2S3 = 2 × 0.001 434 37 ... = 0.002 868 75 … mol
and m(As) = n × M = 0.028 687 5 ... × 74.9 = 0.214 869 … g
%As in the pesticide = (mass of As/mass of pesticide) × 100
= (0.214 869 .../2.15) × 100
= 9.99%
30. (a) silver chloride
(b) n(AgCl) = m/M = 2.359/143.4 = 0.01645 mol
NaCl(aq) + AgNO3(aq) AgCl(s) + NaNO3(aq)
© John Wiley & Sons Australia, Ltd 9
Note that an equation does not have to be written to establish the relationship in mole
between AgCl and NaCl. The fact that there is one Cl in both the AgCl and Nal formulas
clearly establishes a 1 : 1 mole ratio.
n(NaCl) = n(AgCl) = m/M = 0.01645 mol
m(NaCl) = n × M = 0.016 45 × 58.5 = 0.962 g
%NaCl = (mass of NaCl/mass of rock salt) × 100
= (0.962/0.997) × 100 = 96.5%
31. (a) n(BaSO4) = m/M = 0.564/233.4 = 0.002 42 mol
n(S) = n(BaSO4) = 0.002 42 mol
m(S) = n × M = 0.002 42 × 32.1 = 0.0777 g
%S = (mass of S/mass of fertiliser) × 100
= (0.0777/2.322) × 100
= 3.35%
(b) The final result would be greater.
32. (a) None of the ions would form an insoluble precipitate.
(b) silver
(c) None of the ions would form a precipitate.
(d) barium
(e) hydroxide
33. to ensure that all of the water has been removed
34. Precipitates are chosen so that their solubilities are very low. Keeping the volume of
water used during the experiment, including that used to wash the precipitate, to a minimum,
is one way of reducing the chance that some of the precipitate may dissolve. It is possible,
however, that some of the precipitate may dissolve and hence the percentage determined in
any experiment may be a little lower than it should be. This would be a systematic error.
36. C, E, H, A, F, D (Steps B and G are not required.)
37. (a) greater
(b) greater
(c) smaller
(d) smaller
© John Wiley & Sons Australia, Ltd 10
Exam practice questions
1. (a) carbon dioxide, CO2
(b) CaCO3(s) CaO(s) + CO2(g)
(c) m(CaO) = 11.64 g
n(CaO) = m/M = 11.64/56.1 = 0.208 mol
n(CaCO3) = n(CaO) = 0.208 mol
m(CaCO3) = n × M = 0.208 × 100.1 = 20.8 g
%CaCO3 = (20.8/25.00) × 100 = 83.2%
(d) The only solid remaining after the sample has been heated to a constant mass is calcium
oxide.
2. (a) n(Mg2P2O7) = m/M = 4.107/222.6 = 0.018 45 mol
n(P) = 2 × n(Mg2P2O7) = 2 × 0.018 45 = 0.036 90 mol
m(P) = n × M = 0.036 90 × 31.0 = 1.14 g
(b) %P = (1.14/14.298) × 100
= 79.7%
(c) No element other than P also forms a precipitate.
3. (a) n(AgCl) = m/M = 4.463/143.4 = 0.031 12 mol
n(NaCl) = n(AgCl) = 0.031 12 mol
m(NaCl) = n × M = 0.031 12 × 58.5 = 1.82 g
(b) concentration = 1.82/10.0 = 0.182 g L–1
(c) Ag+(aq) + Cl–(aq) AgCl(s)
(d) It is far less likely that any of the AgCl precipitate will dissolve; i.e. it is far more likely
that an accurate result will be obtained.
(e) Chloride ions are the only ions present in the water that form a precipitate with the
silver ions that are added.
© John Wiley & Sons Australia, Ltd 11
You might also like
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Tutorial 1 AnswerDocument15 pagesTutorial 1 Answerd3kamsNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Practice Questions Key - 2022 FallDocument2 pagesPractice Questions Key - 2022 FallFx -No ratings yet
- Homework 6 KeyDocument6 pagesHomework 6 KeyTinh AppleNo ratings yet
- Sample Exam in ChemDocument13 pagesSample Exam in ChemDiane GuilaranNo ratings yet
- Oxford IB Chem Topic 1 AnswersDocument5 pagesOxford IB Chem Topic 1 AnswersJackson LeungNo ratings yet
- Chemistry - ANSWERS - Bylikin, Horner, Murphy and Tarcy - Oxford 2014 PDFDocument100 pagesChemistry - ANSWERS - Bylikin, Horner, Murphy and Tarcy - Oxford 2014 PDFRabia Rafique100% (1)
- Determining The Thermal Behaviour and Composition of Calcium Oxalate MonohydrateDocument11 pagesDetermining The Thermal Behaviour and Composition of Calcium Oxalate Monohydrateadda67% (3)
- Ch123 Exam II Practice Exam Spring2011Document7 pagesCh123 Exam II Practice Exam Spring2011christopher92530% (1)
- Tutorial Sheet 1 - KeyDocument11 pagesTutorial Sheet 1 - KeyRobert SimazuoNo ratings yet
- RD RD RD RD: 6 Theoretical Problems 2 Practical ProblemsDocument13 pagesRD RD RD RD: 6 Theoretical Problems 2 Practical Problemslos sabiosNo ratings yet
- CH 101 Exam I Fall 2012Document7 pagesCH 101 Exam I Fall 2012Simon JesterNo ratings yet
- Tugas Latihan TitrasiDocument9 pagesTugas Latihan TitrasithomasdarmaNo ratings yet
- Chapter3problems-Bursoln 25362232Document11 pagesChapter3problems-Bursoln 25362232leoriza mae salapareNo ratings yet
- Chapter 5: Chemical AccountingDocument9 pagesChapter 5: Chemical AccountingNahed YacoubNo ratings yet
- Worked Solutions To Problems: 1 Acid RainDocument18 pagesWorked Solutions To Problems: 1 Acid RainThiago SantosNo ratings yet
- 1.stoichiometric RelationshipsDocument29 pages1.stoichiometric RelationshipsLaraStrbacNo ratings yet
- Topic-1.1 Formulae, Equations and Amount of SubstancesDocument20 pagesTopic-1.1 Formulae, Equations and Amount of SubstancesAneeka KamalNo ratings yet
- AS Chemistry Answer Sheet 02 AnsDocument10 pagesAS Chemistry Answer Sheet 02 Ansthegreatwardini0% (1)
- Topic 1 Formulae, Equations and Amount of SubstanceDocument25 pagesTopic 1 Formulae, Equations and Amount of SubstanceWhitneyNo ratings yet
- Moles TestDocument5 pagesMoles TestMahedyNo ratings yet
- 第一次期中考考古題Document5 pages第一次期中考考古題林展宏No ratings yet
- Chapter 4Document23 pagesChapter 4Manuela MendozaNo ratings yet
- Cic7ed ch1 Answers PDFDocument4 pagesCic7ed ch1 Answers PDFWaqar AhmadNo ratings yet
- FORM 4 ENERGY CHANGES IN CHEMICAL AND PHYSICAL PROCESSES ANS Teacher - Co - .KeDocument8 pagesFORM 4 ENERGY CHANGES IN CHEMICAL AND PHYSICAL PROCESSES ANS Teacher - Co - .KeCitron AkhalaNo ratings yet
- INJSO Answer Key & SolutionDocument5 pagesINJSO Answer Key & SolutionYatish Goyal100% (1)
- Answers To Quick Questions: Chemistry in Context 6th Edition Answers 1Document4 pagesAnswers To Quick Questions: Chemistry in Context 6th Edition Answers 1John SmithNo ratings yet
- Tugas Latihan TitrasiDocument9 pagesTugas Latihan TitrasithomasdarmaNo ratings yet
- Chapter 5 Review SolutionDocument7 pagesChapter 5 Review SolutionSFDLSFHIOANo ratings yet
- Ch3 ProblemsDocument8 pagesCh3 ProblemsNewshaSajadiNo ratings yet
- Chemical Reactions: Reactants ProductsDocument16 pagesChemical Reactions: Reactants ProductsRSLNo ratings yet
- Chemistry NYA Answers Part 1Document27 pagesChemistry NYA Answers Part 1qwerty514No ratings yet
- Class 9 ScienceChapter 8Document9 pagesClass 9 ScienceChapter 8Lr VarteNo ratings yet
- As Unit 1 Chapter 1 Past PapersDocument20 pagesAs Unit 1 Chapter 1 Past PapersK K Chamath Aachinthya0% (1)
- Therm DynDocument13 pagesTherm DynSumathi SrinivasNo ratings yet
- Test1B S23Document8 pagesTest1B S23Nivedha NatarajNo ratings yet
- Imcho2020s.en 2Document15 pagesImcho2020s.en 2Quốc NguyễnNo ratings yet
- Problem 1 (Author Khvalyuk V.N.) : 54th International Mendeleev Olympiad, 2020 1 Theoretical Tour SolutionsDocument15 pagesProblem 1 (Author Khvalyuk V.N.) : 54th International Mendeleev Olympiad, 2020 1 Theoretical Tour SolutionsQuốc NguyễnNo ratings yet
- HW3 SolnDocument5 pagesHW3 SolnNaury N OliveiraNo ratings yet
- Y1 P2 Summative Topics 1.1 1.2 11.1Document7 pagesY1 P2 Summative Topics 1.1 1.2 11.124zaltayNo ratings yet
- 40 Austrian Chemistry Olympiad National CompetitionDocument17 pages40 Austrian Chemistry Olympiad National CompetitionGerel BayrmagnaiNo ratings yet
- Introductory Chemistry 1St Edition Revell Test Bank Full Chapter PDFDocument34 pagesIntroductory Chemistry 1St Edition Revell Test Bank Full Chapter PDFmatthewelmerwjxqf100% (8)
- Assignment - 1-Mole Concept-AbhimanyuDocument7 pagesAssignment - 1-Mole Concept-Abhimanyuaryan aggarwalNo ratings yet
- DPP For Jee Daily Practice Problems CH 1: Some Basic Concepts of Chemistry SolutionsDocument8 pagesDPP For Jee Daily Practice Problems CH 1: Some Basic Concepts of Chemistry Solutionshcvy7zbjs6No ratings yet
- CHEM 111-Exam 3Document8 pagesCHEM 111-Exam 3emmanuel.olaji0855No ratings yet
- Problem Set PCPDocument4 pagesProblem Set PCPJenny LlanesNo ratings yet
- Helpsheet 4 103Document4 pagesHelpsheet 4 103Uday Prakash SahuNo ratings yet
- CHEM 203 Sample Final ExamDocument7 pagesCHEM 203 Sample Final ExamKhalil FanousNo ratings yet
- Latihan StoikiometriDocument4 pagesLatihan StoikiometriArda RahmainiNo ratings yet
- Full Download Introductory Chemistry Concepts and Critical Thinking Corwin 7Th Edition Solutions Manual PDFDocument45 pagesFull Download Introductory Chemistry Concepts and Critical Thinking Corwin 7Th Edition Solutions Manual PDFamy.lopez138100% (18)
- 2004 - Chimie - Internationala - Solutii - Clasa A XII-a - 0 PDFDocument20 pages2004 - Chimie - Internationala - Solutii - Clasa A XII-a - 0 PDFiugulescu laurentiuNo ratings yet
- ISM Chapter 04Document19 pagesISM Chapter 04宇涵鄒No ratings yet
- Chemistry AssignmentDocument6 pagesChemistry Assignmentakuakwartemaamensah123No ratings yet
- Kneader Mixer Lab Report by Group E (SECTION A)Document16 pagesKneader Mixer Lab Report by Group E (SECTION A)Maryam Fatima100% (1)
- AP Chapter 3b - StoichiometryDocument3 pagesAP Chapter 3b - StoichiometryCacaNo ratings yet
- StiochiometryDocument11 pagesStiochiometryIndrojyoti MondalNo ratings yet
- HKDSE CHEMISTRY - Book 4A AnsDocument48 pagesHKDSE CHEMISTRY - Book 4A AnsSteven Chu100% (1)
- CHEM 1307 Exam 1 Practice Problems: C CL P K NDocument5 pagesCHEM 1307 Exam 1 Practice Problems: C CL P K NPayal PawarNo ratings yet
- Solucoes ICHO28 A ICHO24Document38 pagesSolucoes ICHO28 A ICHO24Leonardo FagundesNo ratings yet
- CH 4 MethodsDocument32 pagesCH 4 MethodsDNo ratings yet
- Chemistry 2 VCE Units 3 and 4 Topic 8 Solutions: Revision QuestionsDocument1 pageChemistry 2 VCE Units 3 and 4 Topic 8 Solutions: Revision QuestionsDNo ratings yet
- 7.07mitosis QuizDocument2 pages7.07mitosis QuizAysha Mohideen50% (2)
- Cell Transport Review WorksheetsDocument8 pagesCell Transport Review WorksheetsD50% (2)
- Topic 1 - Practice TestDocument2 pagesTopic 1 - Practice TestDNo ratings yet
- C 07 Reducing Balance Loans Annuities&PerpetuitiesDocument2 pagesC 07 Reducing Balance Loans Annuities&PerpetuitiesDNo ratings yet
- Meiosis Study SheetDocument3 pagesMeiosis Study SheetDNo ratings yet
- HHD School Assessed Coursework Report 2016Document8 pagesHHD School Assessed Coursework Report 2016DNo ratings yet
- CH 2 MethodsDocument19 pagesCH 2 MethodsDNo ratings yet
- QZ - Cell Membrane and The Parts of The CellDocument2 pagesQZ - Cell Membrane and The Parts of The CellDNo ratings yet
- Maus Study NotesDocument22 pagesMaus Study NotesD100% (2)
- Cell Types: Compare/ Contrast: Directions: With Your Partner, Complete The Venn Diagram That Compares Pictures A and BDocument3 pagesCell Types: Compare/ Contrast: Directions: With Your Partner, Complete The Venn Diagram That Compares Pictures A and BDNo ratings yet
- The Complete Maus - Chapter QuestionsDocument6 pagesThe Complete Maus - Chapter QuestionsD100% (1)
- Immunity Mark SchemeDocument6 pagesImmunity Mark SchemeDNo ratings yet
- Edward Jenner and The History of Smallpox and Vaccination: S R, MD, P DDocument5 pagesEdward Jenner and The History of Smallpox and Vaccination: S R, MD, P DMoisés PonceNo ratings yet
- Lesson 3 Mitosis WorksheetDocument4 pagesLesson 3 Mitosis WorksheetDNo ratings yet
- Immunity Unit 4 Topic 6 WorksheetDocument17 pagesImmunity Unit 4 Topic 6 WorksheetAysha MohideenNo ratings yet
- Helper T CellsDocument6 pagesHelper T CellsDNo ratings yet
- Background Information On The Writer - Peter GoldsworthyDocument4 pagesBackground Information On The Writer - Peter GoldsworthyAngie NguyenNo ratings yet
- Cell Membrane Information WorksheetDocument4 pagesCell Membrane Information WorksheetD100% (1)
- Bone Marrow (Hematopoietic) Stem Cells - Stemcells - NihDocument16 pagesBone Marrow (Hematopoietic) Stem Cells - Stemcells - NihDNo ratings yet
- Linear Relationships HSC Past QuestionsDocument4 pagesLinear Relationships HSC Past QuestionsDNo ratings yet
- Pedigrees WsDocument3 pagesPedigrees WsDNo ratings yet
- VCE Biology Resources 2013-2016: WWW - Vcaa.vic - Edu.au/vce/studies/biology/biologyindex - HTMLDocument8 pagesVCE Biology Resources 2013-2016: WWW - Vcaa.vic - Edu.au/vce/studies/biology/biologyindex - HTMLDNo ratings yet
- 2016 VCE English Examination Report: General CommentsDocument8 pages2016 VCE English Examination Report: General CommentsDNo ratings yet
- LAB GravimetricAnalysisSulfateMixtureDocument3 pagesLAB GravimetricAnalysisSulfateMixtureDNo ratings yet
- Name - Hour - Date - Scatter Plots and Lines of Best Fit WorksheetDocument2 pagesName - Hour - Date - Scatter Plots and Lines of Best Fit WorksheetMCHNo ratings yet
- Stoich 2 PDFDocument1 pageStoich 2 PDFDNo ratings yet
- Direct VariationDocument4 pagesDirect VariationDNo ratings yet
- US2726694 - Single Screw Actuated Pivoted Clamp (Saxton Clamp - Kant-Twist)Document2 pagesUS2726694 - Single Screw Actuated Pivoted Clamp (Saxton Clamp - Kant-Twist)devheadbot100% (1)
- From Assessment To Purchase - A Three-Stage ModelDocument15 pagesFrom Assessment To Purchase - A Three-Stage ModelRONAL EMERSON NOA ORTEGANo ratings yet
- V. Activities: A. Directions: Do The Activity Below. (20 PTS.) Note: Work On This OfflineDocument7 pagesV. Activities: A. Directions: Do The Activity Below. (20 PTS.) Note: Work On This OfflineKeith Neomi CruzNo ratings yet
- Solved - Which $1,000 Bond Has The Higher Yield To Maturity, A T...Document4 pagesSolved - Which $1,000 Bond Has The Higher Yield To Maturity, A T...Sanjna ChimnaniNo ratings yet
- Solution Manual For Introductory Statistics 9th Edition by Mann Chapters 1 13 PDFDocument10 pagesSolution Manual For Introductory Statistics 9th Edition by Mann Chapters 1 13 PDFa40095824643% (14)
- 1.project FullDocument75 pages1.project FullKolliparaDeepakNo ratings yet
- Dielectric Properties of SolidsDocument15 pagesDielectric Properties of SolidsMahesh Lohith K.S100% (11)
- Attention Gated Encoder-Decoder For Ultrasonic Signal DenoisingDocument9 pagesAttention Gated Encoder-Decoder For Ultrasonic Signal DenoisingIAES IJAINo ratings yet
- An4879 Introduction To Usb Hardware and PCB Guidelines Using Stm32 Mcus StmicroelectronicsDocument26 pagesAn4879 Introduction To Usb Hardware and PCB Guidelines Using Stm32 Mcus StmicroelectronicsBulentNo ratings yet
- Servodisc CatalogDocument87 pagesServodisc CatalogEstebanRojasKrustofskyNo ratings yet
- IK Gujral Punjab Technical University: 1. Electric ChargeDocument12 pagesIK Gujral Punjab Technical University: 1. Electric ChargeJashandeep KaurNo ratings yet
- L4 Subdivision of PlotsDocument20 pagesL4 Subdivision of PlotsKenny BoatNo ratings yet
- HydrocarbonsDocument5 pagesHydrocarbonsClaire Danes Tabamo DagalaNo ratings yet
- Projector Spec 8040Document1 pageProjector Spec 8040Radient MushfikNo ratings yet
- CSC:361-Software Engineering: Semester: Fall2020Document39 pagesCSC:361-Software Engineering: Semester: Fall2020hamsfayyazNo ratings yet
- Rotary Valve Functions BookletDocument17 pagesRotary Valve Functions Bookletamahmoud3No ratings yet
- Balancing of Reciprocating MassesDocument74 pagesBalancing of Reciprocating MassesBharaniSai100% (1)
- Periodic Table and AtomsDocument5 pagesPeriodic Table and AtomsShoroff AliNo ratings yet
- P 130881757895329843Document44 pagesP 130881757895329843Vijay MohanNo ratings yet
- Review Skills 1-8Document1 pageReview Skills 1-8TegarNo ratings yet
- Aluminum: DR 900 Analytical ProcedureDocument4 pagesAluminum: DR 900 Analytical Procedurewulalan wulanNo ratings yet
- M. Fatur - H1C018040 - PETROLOGIDocument15 pagesM. Fatur - H1C018040 - PETROLOGIFaturrachmanNo ratings yet
- Ethoxy 1Document77 pagesEthoxy 1HoshiNo ratings yet
- Cep MPDocument1 pageCep MPAzmat HabeebNo ratings yet
- Pressure Sensor Air PST Datasheet 51 en 2780071435Document3 pagesPressure Sensor Air PST Datasheet 51 en 2780071435Luis GuevaraNo ratings yet
- Sqluser v11r1Document199 pagesSqluser v11r1samnolenNo ratings yet
- Como Desarmar Sony Vaio VGN-FE PDFDocument14 pagesComo Desarmar Sony Vaio VGN-FE PDFPeruInalambrico Redes InalambricasNo ratings yet
- PS1Document2 pagesPS1Nitesh Kumar DubeyNo ratings yet
- BSIT Nov Dec 2012 2nd CycleDocument59 pagesBSIT Nov Dec 2012 2nd CyclePiyush PriyankNo ratings yet
- Optimization of Decarbonization On Steel IndustryDocument28 pagesOptimization of Decarbonization On Steel Industrymsantosu000No ratings yet
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaFrom EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaRating: 4.5 out of 5 stars4.5/5 (266)
- Summary: Atomic Habits by James Clear: An Easy & Proven Way to Build Good Habits & Break Bad OnesFrom EverandSummary: Atomic Habits by James Clear: An Easy & Proven Way to Build Good Habits & Break Bad OnesRating: 5 out of 5 stars5/5 (1635)
- The Compound Effect by Darren Hardy - Book Summary: Jumpstart Your Income, Your Life, Your SuccessFrom EverandThe Compound Effect by Darren Hardy - Book Summary: Jumpstart Your Income, Your Life, Your SuccessRating: 5 out of 5 stars5/5 (456)
- Summary of The Anxious Generation by Jonathan Haidt: How the Great Rewiring of Childhood Is Causing an Epidemic of Mental IllnessFrom EverandSummary of The Anxious Generation by Jonathan Haidt: How the Great Rewiring of Childhood Is Causing an Epidemic of Mental IllnessNo ratings yet
- The One Thing: The Surprisingly Simple Truth Behind Extraordinary ResultsFrom EverandThe One Thing: The Surprisingly Simple Truth Behind Extraordinary ResultsRating: 4.5 out of 5 stars4.5/5 (709)
- The Whole-Brain Child by Daniel J. Siegel, M.D., and Tina Payne Bryson, PhD. - Book Summary: 12 Revolutionary Strategies to Nurture Your Child’s Developing MindFrom EverandThe Whole-Brain Child by Daniel J. Siegel, M.D., and Tina Payne Bryson, PhD. - Book Summary: 12 Revolutionary Strategies to Nurture Your Child’s Developing MindRating: 4.5 out of 5 stars4.5/5 (57)
- Summary of 12 Rules for Life: An Antidote to ChaosFrom EverandSummary of 12 Rules for Life: An Antidote to ChaosRating: 4.5 out of 5 stars4.5/5 (294)
- The War of Art by Steven Pressfield - Book Summary: Break Through The Blocks And Win Your Inner Creative BattlesFrom EverandThe War of Art by Steven Pressfield - Book Summary: Break Through The Blocks And Win Your Inner Creative BattlesRating: 4.5 out of 5 stars4.5/5 (273)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessFrom EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessRating: 4.5 out of 5 stars4.5/5 (328)
- Summary of Slow Productivity by Cal Newport: The Lost Art of Accomplishment Without BurnoutFrom EverandSummary of Slow Productivity by Cal Newport: The Lost Art of Accomplishment Without BurnoutRating: 1 out of 5 stars1/5 (1)
- How To Win Friends and Influence People by Dale Carnegie - Book SummaryFrom EverandHow To Win Friends and Influence People by Dale Carnegie - Book SummaryRating: 5 out of 5 stars5/5 (556)
- Can't Hurt Me by David Goggins - Book Summary: Master Your Mind and Defy the OddsFrom EverandCan't Hurt Me by David Goggins - Book Summary: Master Your Mind and Defy the OddsRating: 4.5 out of 5 stars4.5/5 (383)
- Essentialism by Greg McKeown - Book Summary: The Disciplined Pursuit of LessFrom EverandEssentialism by Greg McKeown - Book Summary: The Disciplined Pursuit of LessRating: 4.5 out of 5 stars4.5/5 (187)
- Summary of The Algebra of Wealth by Scott Galloway: A Simple Formula for Financial SecurityFrom EverandSummary of The Algebra of Wealth by Scott Galloway: A Simple Formula for Financial SecurityNo ratings yet
- Summary of Atomic Habits by James ClearFrom EverandSummary of Atomic Habits by James ClearRating: 5 out of 5 stars5/5 (169)
- How Not to Die by Michael Greger MD, Gene Stone - Book Summary: Discover the Foods Scientifically Proven to Prevent and Reverse DiseaseFrom EverandHow Not to Die by Michael Greger MD, Gene Stone - Book Summary: Discover the Foods Scientifically Proven to Prevent and Reverse DiseaseRating: 4.5 out of 5 stars4.5/5 (83)
- Designing Your Life by Bill Burnett, Dave Evans - Book Summary: How to Build a Well-Lived, Joyful LifeFrom EverandDesigning Your Life by Bill Burnett, Dave Evans - Book Summary: How to Build a Well-Lived, Joyful LifeRating: 4.5 out of 5 stars4.5/5 (62)
- Steal Like an Artist by Austin Kleon - Book Summary: 10 Things Nobody Told You About Being CreativeFrom EverandSteal Like an Artist by Austin Kleon - Book Summary: 10 Things Nobody Told You About Being CreativeRating: 4.5 out of 5 stars4.5/5 (128)
- Summary, Analysis, and Review of Daniel Kahneman's Thinking, Fast and SlowFrom EverandSummary, Analysis, and Review of Daniel Kahneman's Thinking, Fast and SlowRating: 3.5 out of 5 stars3.5/5 (2)
- Blink by Malcolm Gladwell - Book Summary: The Power of Thinking Without ThinkingFrom EverandBlink by Malcolm Gladwell - Book Summary: The Power of Thinking Without ThinkingRating: 4.5 out of 5 stars4.5/5 (114)
- The 5 Second Rule by Mel Robbins - Book Summary: Transform Your Life, Work, and Confidence with Everyday CourageFrom EverandThe 5 Second Rule by Mel Robbins - Book Summary: Transform Your Life, Work, and Confidence with Everyday CourageRating: 4.5 out of 5 stars4.5/5 (329)
- Summary of The Galveston Diet by Mary Claire Haver MD: The Doctor-Developed, Patient-Proven Plan to Burn Fat and Tame Your Hormonal SymptomsFrom EverandSummary of The Galveston Diet by Mary Claire Haver MD: The Doctor-Developed, Patient-Proven Plan to Burn Fat and Tame Your Hormonal SymptomsNo ratings yet
- We Were the Lucky Ones: by Georgia Hunter | Conversation StartersFrom EverandWe Were the Lucky Ones: by Georgia Hunter | Conversation StartersNo ratings yet
- Make It Stick by Peter C. Brown, Henry L. Roediger III, Mark A. McDaniel - Book Summary: The Science of Successful LearningFrom EverandMake It Stick by Peter C. Brown, Henry L. Roediger III, Mark A. McDaniel - Book Summary: The Science of Successful LearningRating: 4.5 out of 5 stars4.5/5 (55)
- Sell or Be Sold by Grant Cardone - Book Summary: How to Get Your Way in Business and in LifeFrom EverandSell or Be Sold by Grant Cardone - Book Summary: How to Get Your Way in Business and in LifeRating: 4.5 out of 5 stars4.5/5 (86)
- Summary: Educated: A Memoir by Tara Westover: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: Educated: A Memoir by Tara Westover: Key Takeaways, Summary & Analysis IncludedRating: 4.5 out of 5 stars4.5/5 (65)
- Psycho-Cybernetics by Maxwell Maltz - Book SummaryFrom EverandPsycho-Cybernetics by Maxwell Maltz - Book SummaryRating: 4.5 out of 5 stars4.5/5 (91)
- Tiny Habits by BJ Fogg - Book Summary: The Small Changes That Change EverythingFrom EverandTiny Habits by BJ Fogg - Book Summary: The Small Changes That Change EverythingRating: 4.5 out of 5 stars4.5/5 (111)