Professional Documents
Culture Documents
Residence Time (Min)
Uploaded by
Hugh Lambert MalinaoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Residence Time (Min)
Uploaded by
Hugh Lambert MalinaoCopyright:
Available Formats
0.
0.5
0.4
Conversion XNaOH
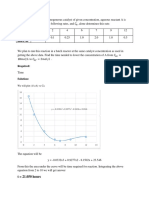
y = -0.0879x + 0.589
0.3 R² = 0.7305
0.2
0.1
0
0 0.5 1 1.5 2 2.5
Residence time (min)
The graph above shows the relationship of the conversion of NaOH with respect to residence time.
Space time is the time required to process one reactor volume of feed while residence time is the time
required for one material to flow in the reactor. For a constant density system, the space time τ is equal to
the residence time 𝑡̅ and is related to volume of the reactor in the equation below.
𝑉𝑃𝐹𝑅
𝜏 = 𝑡̅ =
∅𝑉,𝑖𝑛
The graph above shows that the conversion of NaOH decreases as residence time increase. However,
theoretically, this should not be the case. A conversion only occurs when molecules collide to react as stated
by the collision model (Zumdahl & Zumdahl, 2012). Increasing residence time allows more possible collisions,
therefore it is expected that, as the residence time of a material in a reactor increases, the conversion of a
reactant increases. This could also be supported by the characteristic equation of a plug flow reactor below.
𝐶𝑁𝑎𝑂𝐻 𝑋𝑁𝑎𝑂𝐻
𝑑𝐶𝑁𝑎𝑂𝐻 𝑑𝑋𝑁𝑎𝑂𝐻
𝜏 = 𝑡̅ = − ∫ =∫
𝐶𝑁𝑎𝑂𝐻 ,𝑖𝑛𝑖𝑡 −𝑟𝑁𝑎𝑂𝐻 0 −𝑟𝑁𝑎𝑂𝐻
From the equation, it can be seen that the residence time is proportional to the conversion of the reactant.
This also suggest that increasing the time of one material or processing of one reactor volume of feed
should increase the conversion. In addition to this, it should also be expected that the conversion increases
with residence time until such time that the conversion a reaches maximum and plateaus.
You might also like
- Experiment 4: Effect of Concentration and Temperature On Rate of Reaction (Dissappearing Cross)Document24 pagesExperiment 4: Effect of Concentration and Temperature On Rate of Reaction (Dissappearing Cross)Malini RajeshNo ratings yet
- Cape Chemistry Unit 1Document22 pagesCape Chemistry Unit 1Audi Sweetangel100% (1)
- CH6711 CRE Lab Manual 3.3.2020 1 To 8 Experiments - Extract - 1-8,12-19Document16 pagesCH6711 CRE Lab Manual 3.3.2020 1 To 8 Experiments - Extract - 1-8,12-19Vignesh Raja.PNo ratings yet
- Kinetics Study of Saponification Reaction in Tubular FlowDocument8 pagesKinetics Study of Saponification Reaction in Tubular FlowhuonpatrickcrollNo ratings yet
- 2046chapter14 PDFDocument22 pages2046chapter14 PDFAsif AliNo ratings yet
- Reaction Kinetics: Continuous Vs Initial Rate DataDocument16 pagesReaction Kinetics: Continuous Vs Initial Rate DatashowhardoNo ratings yet
- Batch Reactor Manual PDFDocument4 pagesBatch Reactor Manual PDFPradeep DiwakarNo ratings yet
- 2.mass Transfer With - Without Chemical ReactionDocument7 pages2.mass Transfer With - Without Chemical ReactionAjeet KumarNo ratings yet
- Cre Lab 9Document3 pagesCre Lab 9Fahad kamranNo ratings yet
- CHM271 - Chapter 5 Chemical Kinetics (Part 1-3)Document40 pagesCHM271 - Chapter 5 Chemical Kinetics (Part 1-3)happyflowerNo ratings yet
- Factors Affecting Reaction RateDocument29 pagesFactors Affecting Reaction RateIna Chiu100% (1)
- Frenkel 94 Determination Diagrams YukawaDocument5 pagesFrenkel 94 Determination Diagrams YukawaJose RamirezNo ratings yet
- Experiment 6: Chemical KineticsDocument28 pagesExperiment 6: Chemical KineticsBalqees HasanNo ratings yet
- Chemical Reaction: (Batch Reactor)Document15 pagesChemical Reaction: (Batch Reactor)Ahmed ZakariaNo ratings yet
- Chemical Kinetics: The Rates and Mechanisms of Chemical ReactionsDocument21 pagesChemical Kinetics: The Rates and Mechanisms of Chemical ReactionsOyinkansola OsiboduNo ratings yet
- Chemical Kinetics-MEDocument22 pagesChemical Kinetics-MEprishaNo ratings yet
- CSTR 40LDocument12 pagesCSTR 40LMohamad SyamilNo ratings yet
- Auto Catalytic Reactions PresentationDocument15 pagesAuto Catalytic Reactions PresentationAmad AmadNo ratings yet
- Chemical KineticsDocument9 pagesChemical KineticsMikey Bryant BonbonNo ratings yet
- Reaction KineticsDocument15 pagesReaction KineticsSadiaShoaibNo ratings yet
- Lab Report TemplateDocument6 pagesLab Report Templatecgjp120391No ratings yet
- Chem 17 - Chemical KineticsDocument6 pagesChem 17 - Chemical KineticsWilfredo LlanaNo ratings yet
- Experiment 1B - Tubular ReactorDocument14 pagesExperiment 1B - Tubular ReactorNajmul Puda PappadamNo ratings yet
- Soal Chapter 21Document5 pagesSoal Chapter 2121-096 Kharisma Theresia Adelina ButarButarNo ratings yet
- Exp 4Document27 pagesExp 4Dhiyyah MardhiyyahNo ratings yet
- Batch ReactorDocument7 pagesBatch ReactorJubell MogoteNo ratings yet
- RatesDocument22 pagesRatesPeterNo ratings yet
- Tarea 2 de Reacciones 2Document14 pagesTarea 2 de Reacciones 2Sait Marcos Orihuela OrihuelaNo ratings yet
- CSTR ManualDocument11 pagesCSTR ManualMelly FulaNo ratings yet
- CreDocument8 pagesCreBHOWMICK PATIDAR 15BCH0085No ratings yet
- Cre PDFDocument8 pagesCre PDFBHOWMICK PATIDAR 15BCH0085No ratings yet
- Reactors1 9Document4 pagesReactors1 9Mourad kharbachNo ratings yet
- Saponification Reaction of Sodium Hydroxide An Ethyl Acetate in A Continuous Stirred Tank Reactor CSTRDocument21 pagesSaponification Reaction of Sodium Hydroxide An Ethyl Acetate in A Continuous Stirred Tank Reactor CSTRsyedmuhammadtarique100% (1)
- Introduction Mass Transfer Single Use Bioreactors White PaperDocument11 pagesIntroduction Mass Transfer Single Use Bioreactors White PaperAna Karen Calderon OrdazNo ratings yet
- Dynamics and Control of Recycle Systems. 2. Comparison Alternative Process DesignsDocument11 pagesDynamics and Control of Recycle Systems. 2. Comparison Alternative Process DesignsJean Pierre León BravoNo ratings yet
- Chem 17 - KINETICS OF THE REACTION BETWEEN S2O3 2 - AND H+Document6 pagesChem 17 - KINETICS OF THE REACTION BETWEEN S2O3 2 - AND H+Wilfredo LlanaNo ratings yet
- S.no. Name of The Experiment Date of Conduction Date of Submission P1 Coil Type Plug Flow Reactor February 2, 2021 February 4, 2021Document14 pagesS.no. Name of The Experiment Date of Conduction Date of Submission P1 Coil Type Plug Flow Reactor February 2, 2021 February 4, 2021DEEPSHIKA DUTTANo ratings yet
- Batch Reactor ExpDocument12 pagesBatch Reactor ExpJack AndreasNo ratings yet
- IIT-JEE Syllabus: RSM79 Ph-II CK CH 1Document69 pagesIIT-JEE Syllabus: RSM79 Ph-II CK CH 1Kushagra SinghNo ratings yet
- CHM271 - Chapter 5 Chemical KineticsDocument79 pagesCHM271 - Chapter 5 Chemical KineticshappyflowerNo ratings yet
- T2 - E5-Phase Transfer CatalystDocument14 pagesT2 - E5-Phase Transfer Catalystabhisheknemade9765No ratings yet
- Lab 05Document29 pagesLab 05zzrot1No ratings yet
- Experiment CSTR 40LDocument18 pagesExperiment CSTR 40LSaber Minato Azrul100% (2)
- Aquino Lab06Document25 pagesAquino Lab06Ai RahNo ratings yet
- Reaction Rates & Chemical EquilibriumDocument29 pagesReaction Rates & Chemical Equilibriumkyla dianneNo ratings yet
- Ideal R Eactors: 2.1 G Eneralised R Eactor M Ass BalanceDocument11 pagesIdeal R Eactors: 2.1 G Eneralised R Eactor M Ass BalanceJonathan ByamunguNo ratings yet
- Kinetics (Gjjkkkgty)Document5 pagesKinetics (Gjjkkkgty)Chrysler Kane DepnagNo ratings yet
- Results and Discussion Report On EXPERIMENT NO. 3 Kinetics of Reaction Between Thiosulfate and Hydrochloric AcidDocument3 pagesResults and Discussion Report On EXPERIMENT NO. 3 Kinetics of Reaction Between Thiosulfate and Hydrochloric AcidNashDanielSilava100% (4)
- CH4002 L12-16 2017 KH Chemical Kinetics IntroductionDocument56 pagesCH4002 L12-16 2017 KH Chemical Kinetics Introductionjesuslovers3000No ratings yet
- Rate of ReactionDocument27 pagesRate of ReactionShofwa AnnisaaNo ratings yet
- Ders 14 Chemical Kinetics PDFDocument25 pagesDers 14 Chemical Kinetics PDFÖmer ErcanNo ratings yet
- Solvolysis LabDocument5 pagesSolvolysis LabAriesNo ratings yet
- PFRDocument19 pagesPFRKangae IlhamNo ratings yet
- Exemple 3 Reactor BATCH SacarosaDocument30 pagesExemple 3 Reactor BATCH Sacarosakatrina SamirNo ratings yet
- Reactor EnggDocument75 pagesReactor EnggarunperthNo ratings yet
- PRT 140 Physical Chemistry Programme Industrial Chemical Process SEM 1 2013/2014Document72 pagesPRT 140 Physical Chemistry Programme Industrial Chemical Process SEM 1 2013/2014Anusia ThevendaranNo ratings yet
- Topic 6/16 Kinetics (Rates of Reactions)Document14 pagesTopic 6/16 Kinetics (Rates of Reactions)Oyinkansola OsiboduNo ratings yet
- ACFrOgBlHk5jHSMGYEBt3FvRn7Qxcx5ceMy1IN9RJthH3lpPhjTUmCAcpbrtggFhnN2wjlOpL CA8PLiPDDVWSix2 Oh3ZZeIOOW0g GOsFvOvbg85Q8S QdXGlyudtryzJT8cdY OepRiAAcbj5Document7 pagesACFrOgBlHk5jHSMGYEBt3FvRn7Qxcx5ceMy1IN9RJthH3lpPhjTUmCAcpbrtggFhnN2wjlOpL CA8PLiPDDVWSix2 Oh3ZZeIOOW0g GOsFvOvbg85Q8S QdXGlyudtryzJT8cdY OepRiAAcbj5مصطفى أحمد عبد الرزاق هاديNo ratings yet
- Vacuum Distillation 2Document3 pagesVacuum Distillation 2Hugh Lambert MalinaoNo ratings yet
- Ethics Case StudyDocument6 pagesEthics Case StudyHugh Lambert MalinaoNo ratings yet
- Lambert's Life: by Hugh Lambert L. MalinaoDocument14 pagesLambert's Life: by Hugh Lambert L. MalinaoHugh Lambert MalinaoNo ratings yet
- Protist: Protozoa-Animal-LikeDocument2 pagesProtist: Protozoa-Animal-LikeHugh Lambert MalinaoNo ratings yet