Professional Documents
Culture Documents
Mitochondria Week 7
Uploaded by
Aamna0 ratings0% found this document useful (0 votes)
34 views3 pagesnotes
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentnotes
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
34 views3 pagesMitochondria Week 7
Uploaded by
Aamnanotes
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 3
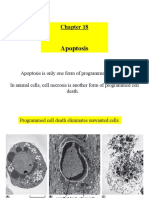
Types of cell death
Two main ways in which cells can die:
1. Necrosis: (uncontrolled cell death). Associated with disease.
2. Apoptosis (programmed cell death or cell suicide). Apoptosis is an
essential part of normal health and development. In an average adult
between 50 and 70 billion cells die through apoptosis per day. In a year
your entire body weight of cells will have died through apoptosis.
Importance of apoptosis
1. Immune system – destroying self-reacting immune cells.
2. Immune system – destroying virus infected cells.
3. Homeostasis – as a counter-balance to cell division and removal of old or
damaged cells.
Cancer – radiotherapy and most chemotherapy drugs work by inducing
apoptosis. Many cancer cells have defects in the apoptosis pathways that make
them resistant to apoptosis.
How is apoptosis triggered?
Two main pathways for triggering apoptosis:
1. Receptor mediated (extrinsic pathway)
2. Mitochondria mediated (intrinsic pathway). The intrinsic pathway can be
activated by a variety of cell stresses such as free radical damage, DNA
damage, viral infection, or loss of survival signals.
Both pathways induce apoptosis by activating a class of proteases in the cell
called caspases.
Caspases - cysteine-aspartic proteases.
A family of 12 proteases that exist as inactive pro-enzymes in cells.
Following activation by cleavage they can activate other caspases in a cascade.
There are two types of apoptotic caspases:
1. Initiator caspases: activate other caspases.
2. Effector (executioner) caspases: break down cellular components such as
the cytoskeleton and DNA.
Activation of caspases is the point of no return in the process of apoptosis.
Role of mitochondria in apoptosis:
Cytochrome C is located in the inner mitochondrial membrane and is an
essential component of the electron transport chain.
In the intrinsic apoptosis pathway, pores form in the outer mitochondrial
membrane allowing release of cytochrome C into the cytosol. Since
cytochrome C is normally only found in the mitochondria, its release into the
cytosol acts as a trigger for apoptosis. Cytochrome C binds to other cytosolic
proteins to form a multi-protein complex called the apoptosome.
The formation of the apoptosome requires cytochrome C, a protein called
Apaf-1, pro-caspase 9 and ATP.
There are 7 molecules of each protein in the complete apoptosome with a
combined molecular weight of 700 KDa.
The end result is the cleavage and therefore activation of pro-caspase 9 into
active caspase 9, an initiator caspase.
What controls the release of cytochrome C from the mitochondria?
The Bcl-2 family of proteins is responsible for the regulation of cytochrome C
release from the mitochondria. There are around 20 members of the family
and consist of both pro-apoptotic and anti-apoptotic proteins. Their ability to
induce apoptosis depends on the balance between the two types.
The Bcl-2 family of proteins is responsible for the regulation of cytochrome C
release from the mitochondria. There are around 20 members of the family
and consist of both pro-apoptotic and anti-apoptotic proteins. Their ability to
induce apoptosis depends on the balance between the two types.
Anti-apoptotic members are thought to exist in the mitochondrial outer
membrane and act to block the action of the pro-apoptotic members.
You might also like
- RH PATHO2022 - (Chapt 02 - Cell Injury, Death, Adaptations - C)Document10 pagesRH PATHO2022 - (Chapt 02 - Cell Injury, Death, Adaptations - C)Toka AlzoubiNo ratings yet
- English 202C Technical DescriptionDocument4 pagesEnglish 202C Technical Descriptiondns5130No ratings yet
- L 18 Apoptosis CM Sp15Document32 pagesL 18 Apoptosis CM Sp15cactus life foreverNo ratings yet
- Chapter 11-ApoptosisDocument26 pagesChapter 11-ApoptosisKw ChanNo ratings yet
- Unit 3 Evading Apoptosis and Telomere DysregulationDocument38 pagesUnit 3 Evading Apoptosis and Telomere DysregulationVidhi RanaNo ratings yet
- New Microsoft Word DocumentDocument4 pagesNew Microsoft Word DocumentsamiunNo ratings yet
- Apoptosis: MBBS, FCPS, M.Phil. Histopathology Department of Pathology CMHLMCDocument42 pagesApoptosis: MBBS, FCPS, M.Phil. Histopathology Department of Pathology CMHLMCAiza AnwarNo ratings yet
- Molecular Basis of ApoptosisDocument14 pagesMolecular Basis of ApoptosisVRUSHTI MEHTANo ratings yet
- The Role of Mitochondria in Apoptosis - PMCDocument34 pagesThe Role of Mitochondria in Apoptosis - PMCAnumol LoranceNo ratings yet
- BIO202!13!14 ApoptosisDocument46 pagesBIO202!13!14 ApoptosisAbhilash Kumar MuthuramanNo ratings yet
- Apoptosis: Ashok Kumar HGDocument15 pagesApoptosis: Ashok Kumar HGVINE TUBENo ratings yet
- Bcl-2 Family ProteinsDocument25 pagesBcl-2 Family Proteinsማላያላም ማላያላም100% (1)
- ApoptasisDocument4 pagesApoptasisDebaraj RoyNo ratings yet
- ApoptosisDocument13 pagesApoptosisAneeza AhmadNo ratings yet
- 1 Apoptosis DesDocument7 pages1 Apoptosis DessashiwiiNo ratings yet
- The Biology of Apoptosis: Fouad Boulos, MD August 2010Document4 pagesThe Biology of Apoptosis: Fouad Boulos, MD August 2010ans11No ratings yet
- Inflammation and Neoplasia ApoptosisDocument6 pagesInflammation and Neoplasia ApoptosisTapitNo ratings yet
- Types of Cell DeathDocument7 pagesTypes of Cell DeathRutvi ChovatiyaNo ratings yet
- ApoptosisDocument2 pagesApoptosisariwodola daramolaNo ratings yet
- Apoptosis Role and Significance in Seminiferous Epithelium: Dr.M.M.Misro Professor Department of Reproductive BiomedicineDocument54 pagesApoptosis Role and Significance in Seminiferous Epithelium: Dr.M.M.Misro Professor Department of Reproductive BiomedicineLinguumNo ratings yet
- ApoptosisDocument3 pagesApoptosisاسماء زياد عبدالجبارNo ratings yet
- Chapter 18 SummaryDocument7 pagesChapter 18 SummaryCharlotteNo ratings yet
- EC13 Cell Ageing DNA TextDocument8 pagesEC13 Cell Ageing DNA TextSilvito94No ratings yet
- Lecture 6 (Finals)Document20 pagesLecture 6 (Finals)larakuzgunNo ratings yet
- Apoptosis Tutorial NotesDocument8 pagesApoptosis Tutorial NotesismealNo ratings yet
- Apoptosis 1Document35 pagesApoptosis 1THANISHTA KUMARNo ratings yet
- APOPTOSISDocument24 pagesAPOPTOSISRick Ghosh (Shreyam)No ratings yet
- Apoptosis and CancerDocument20 pagesApoptosis and CancerSajjad Ahmad0% (1)
- External Pathway of ApoptosisDocument2 pagesExternal Pathway of ApoptosisZoya LiaquatNo ratings yet
- Chapter 18 - Apoptosis - 112612Document28 pagesChapter 18 - Apoptosis - 112612Anonymous nkR1PhIHNo ratings yet
- Apoptosis, GENERAL PATHOLOGYDocument16 pagesApoptosis, GENERAL PATHOLOGYAhmad100% (1)
- Cell Death - Mechanisms: K Siva PrasadDocument59 pagesCell Death - Mechanisms: K Siva PrasadnaimNo ratings yet
- Apoptosis and CancerDocument29 pagesApoptosis and CancerSajjad AhmadNo ratings yet
- Leaflet Waspada DiareDocument10 pagesLeaflet Waspada DiareNikkiNo ratings yet
- Apoptosis: Muhammad ShoaibDocument56 pagesApoptosis: Muhammad ShoaibMuhammad ShoaibNo ratings yet
- Apoptosis: Jalal Ghasemzadeh Andrology LabDocument67 pagesApoptosis: Jalal Ghasemzadeh Andrology LabValeria DezaNo ratings yet
- Apoptosis and CancerDocument20 pagesApoptosis and CancerpriyaaNo ratings yet
- Cancer BiologyDocument14 pagesCancer BiologyADARSH RNo ratings yet
- Cell Cycle (Apoptosis)Document11 pagesCell Cycle (Apoptosis)Siyad AdanNo ratings yet
- DR Shazia RashidDocument21 pagesDR Shazia RashidShubham agrayNo ratings yet
- ApoptosisDocument5 pagesApoptosisHenry LauNo ratings yet
- Apoptosis: Hussam Telfah, MBBS, FrcpathDocument24 pagesApoptosis: Hussam Telfah, MBBS, Frcpathبراءة أحمد السلاماتNo ratings yet
- Programmed Cell Death: (Apoptosis)Document49 pagesProgrammed Cell Death: (Apoptosis)Abirami SankarNo ratings yet
- Apoptosis: Proliferation and Physiological Cell Death, Apoptosis. Both of These ProcessesDocument3 pagesApoptosis: Proliferation and Physiological Cell Death, Apoptosis. Both of These ProcesseswaheedkabNo ratings yet
- ApoptosisDocument3 pagesApoptosiskatende WilsonNo ratings yet
- Pathology خالد طارق موددDocument1 pagePathology خالد طارق موددKhaled ModadNo ratings yet
- Don't Think of Death As An Ending. Think of It As A Really Effective Way of Cutting Down Your Expenses.Document51 pagesDon't Think of Death As An Ending. Think of It As A Really Effective Way of Cutting Down Your Expenses.magician09876No ratings yet
- ApoptosisDocument20 pagesApoptosisRushikesh Rahul KadamNo ratings yet
- Growth Factors & Cell Death...Document22 pagesGrowth Factors & Cell Death...siddhi meenaNo ratings yet
- Tools & Tips For Analyzing Apoptosis: A Kit Selection GuideDocument40 pagesTools & Tips For Analyzing Apoptosis: A Kit Selection GuideCatherineYeohNo ratings yet
- Intercellular Communications & ApoptosisDocument26 pagesIntercellular Communications & ApoptosisAjinkya AravindNo ratings yet
- Biochemistry of Apoptotic Cell Death: Acta Pharm. 53 (2003) 151-164Document14 pagesBiochemistry of Apoptotic Cell Death: Acta Pharm. 53 (2003) 151-164Gayathri MaigandanNo ratings yet
- Tools & Tips For Analyzing Apoptosis: A Kit Selection GuideDocument40 pagesTools & Tips For Analyzing Apoptosis: A Kit Selection GuideexecNo ratings yet
- Apoptosis: Dr. Prabesh K Choudhary Final Year Resident MD PathologyDocument47 pagesApoptosis: Dr. Prabesh K Choudhary Final Year Resident MD PathologyAhmed BioNo ratings yet
- Mitochondrial ApoptosisDocument5 pagesMitochondrial ApoptosisRuheen MariamNo ratings yet
- Apoptosis: Jalal Ghasemzadeh Andrology LabDocument67 pagesApoptosis: Jalal Ghasemzadeh Andrology LabmkmanNo ratings yet
- Apoptosis PDFDocument67 pagesApoptosis PDFmkmanNo ratings yet
- Apoptosis PDFDocument67 pagesApoptosis PDFmkman100% (1)
- Human Chromosomes: An Illustrated Introduction to Human CytogeneticsFrom EverandHuman Chromosomes: An Illustrated Introduction to Human CytogeneticsRating: 5 out of 5 stars5/5 (1)
- STAT 713 Mathematical Statistics Ii: Lecture NotesDocument152 pagesSTAT 713 Mathematical Statistics Ii: Lecture NotesLiban Ali MohamudNo ratings yet
- Speaking With Confidence: Chapter Objectives: Chapter OutlineDocument12 pagesSpeaking With Confidence: Chapter Objectives: Chapter OutlinehassanNo ratings yet
- Food and Beverages Sample Script For NCADocument11 pagesFood and Beverages Sample Script For NCAHONEY ROSE NAKILANo ratings yet
- Practice Test 1 + 2Document19 pagesPractice Test 1 + 2yếnNo ratings yet
- Post Graduate Diploma in Psychological CounselingDocument1 pagePost Graduate Diploma in Psychological CounselingAvalokiteswari KurupNo ratings yet
- Cosmetic-Regulations, Research & Marketing Challenges and Global Compliance: An OverviewDocument19 pagesCosmetic-Regulations, Research & Marketing Challenges and Global Compliance: An Overviewmaria sepulvedaNo ratings yet
- Essay, How Microscopes Have Contributed To Our Understanding of Living OrganismsDocument2 pagesEssay, How Microscopes Have Contributed To Our Understanding of Living Organismslinanqikiki82% (11)
- Animal Cells PDFDocument4 pagesAnimal Cells PDFFalah HabibNo ratings yet
- Object: Annex A, B, C DDocument74 pagesObject: Annex A, B, C DfjsdNo ratings yet
- Raw Material Chemical AnalysisDocument41 pagesRaw Material Chemical AnalysisVinod Kumar VermaNo ratings yet
- S01 Hydraulic and Eletric DiagramDocument18 pagesS01 Hydraulic and Eletric DiagramgadeharogNo ratings yet
- Derivation of Gravity Loads PDFDocument4 pagesDerivation of Gravity Loads PDFHenry TuganoNo ratings yet
- Rossmann Repair Training Guide - Google SlidesDocument167 pagesRossmann Repair Training Guide - Google Slidesmirza baigNo ratings yet
- HW - MainlineList - 2023 - FINAL 2 17 23 UPDATEDDocument9 pagesHW - MainlineList - 2023 - FINAL 2 17 23 UPDATEDJosé Mario González AlfaroNo ratings yet
- Question Bank For Chapter#6Document11 pagesQuestion Bank For Chapter#6krishnam rajuNo ratings yet
- Standard Safety Practices Manual PDFDocument350 pagesStandard Safety Practices Manual PDFsithulibraNo ratings yet
- Defeat Cancer NaturallyDocument94 pagesDefeat Cancer NaturallyRknuviprasys Low100% (3)
- Grade 11 Learning GuideDocument28 pagesGrade 11 Learning GuideMary-Rose Casuyon100% (1)
- Aakash Zoology Study Package 3 SolutionsssssssDocument104 pagesAakash Zoology Study Package 3 SolutionsssssssRishika PaulNo ratings yet
- NCERT Class 7 English Part 1 PDFDocument157 pagesNCERT Class 7 English Part 1 PDFVvs SadanNo ratings yet
- Related Literature BioplasticsDocument19 pagesRelated Literature BioplasticsJames LimNo ratings yet
- Aeration PaperDocument11 pagesAeration PapersehonoNo ratings yet
- 2018-Me-184 MMDocument28 pages2018-Me-184 MMKhizer Nauman RanaNo ratings yet
- Underground-Sprayed Concrete BrochureDocument12 pagesUnderground-Sprayed Concrete BrochureEngTamerNo ratings yet
- CPower Product Training.09.2016.EnDocument70 pagesCPower Product Training.09.2016.Enerdinc100% (1)
- High Voltage Fast-Switching NPN Power Transistor: FeaturesDocument11 pagesHigh Voltage Fast-Switching NPN Power Transistor: FeaturesVESVOCNo ratings yet
- Unidajump2019,+5 +31-42+JP+9 (1) +April+2018+AminullahDocument12 pagesUnidajump2019,+5 +31-42+JP+9 (1) +April+2018+AminullahSatria MandalaNo ratings yet
- Taenia SoliumDocument40 pagesTaenia SoliumBio SciencesNo ratings yet
- RediFlex Hoses Data SheetDocument2 pagesRediFlex Hoses Data SheetNordson Adhesive Dispensing SystemsNo ratings yet
- Project Sanjay YadavDocument51 pagesProject Sanjay YadavriyacomputerNo ratings yet