Professional Documents
Culture Documents
Analysis of Bleach by Iodometry
Uploaded by
lar-sen0 ratings0% found this document useful (0 votes)
972 views2 pagesCopyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
972 views2 pagesAnalysis of Bleach by Iodometry
Uploaded by
lar-senCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
Analysis of Bleach by Iodometry
Objective: A) to prepare and standardize a solution of Na₂S₂O₃ -5H₂O using KBrO₃ as a primary standard.
B) To use the standardized Na₂S₂O₃ solution to determine the concentration of OCl¯ in a
bleach sample.
Equations: Part A:
1) BrO₃¯ + I¯ Br¯ + I₂
2) I₂ + S₂O₃¯² S₄O₆ +I¯
Part B:
1) ClO¯ +I¯ Cl¯ + I₂
2) I₂ + S₂O₃¯² I¯ + S₄O₆¯²
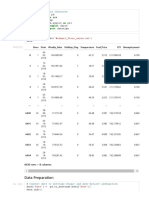
Data Table:
Part 1
Trial Initial Midpoint Endpoint
1 50.0mL 23.2mL 23.7mL
2 50.0mL 25.6mL 26.3mL
3 50.0mL 24.1mL 24.5mL
Avg= 25.07mL
Part 2
Tria Initial Midpoint Endpoint
l
1 50.0mL 38.0mL 40.09mL
2 50.0mL 38.30mL 39.98mL
3 50.0mL 38.59mL 39.69mL
Avg= 9.81
Calculations:
1) moles=ML
Moles= (2.00x10¯²M) (25.07mL)
=0.5014moles
2)
3)
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Lab ManualDocument69 pagesLab ManualPradeepNo ratings yet
- 3D CL Correction S1223RTLDocument7 pages3D CL Correction S1223RTLakatsuki.exeNo ratings yet
- Research Methods SESSIONS STUDENTS Abeeku PDFDocument287 pagesResearch Methods SESSIONS STUDENTS Abeeku PDFdomaina2008100% (3)
- Siemens - Microsoft Hyper V Case StudyDocument2 pagesSiemens - Microsoft Hyper V Case StudyPaul AdamsNo ratings yet
- Mission Statement Generator WorksheetDocument9 pagesMission Statement Generator WorksheetMohamed SururrNo ratings yet
- Abstract - Freezing Point Depression Is ADocument5 pagesAbstract - Freezing Point Depression Is AMinahNo ratings yet
- Certification of Anti-Seismic Devices According To The European Standard EN 15129:2009: Tasks For Manufacturers and Notified BodiesDocument9 pagesCertification of Anti-Seismic Devices According To The European Standard EN 15129:2009: Tasks For Manufacturers and Notified BodiesRobby PermataNo ratings yet
- Retail Analysis WalmartDocument18 pagesRetail Analysis WalmartNavin MathadNo ratings yet
- Pts English Kelas XDocument6 pagesPts English Kelas XindahNo ratings yet
- Journal of Teacher Education-2008-Osguthorpe-288-99 PDFDocument13 pagesJournal of Teacher Education-2008-Osguthorpe-288-99 PDFFauzan WildanNo ratings yet
- Jurnal Internasional Tentang ScabiesDocument9 pagesJurnal Internasional Tentang ScabiesGalyNo ratings yet
- PreliminaryDocument65 pagesPreliminarysame.pxtNo ratings yet
- Advanced Java SlidesDocument134 pagesAdvanced Java SlidesDeepa SubramanyamNo ratings yet
- 17. ĐỀ SỐ 17 HSG ANH 9 HUYỆNDocument9 pages17. ĐỀ SỐ 17 HSG ANH 9 HUYỆNHồng Hoàn NguyễnNo ratings yet
- Chimney Design UnlineDocument9 pagesChimney Design Unlinemsn sastryNo ratings yet
- CP100 Module 2 - Getting Started With Google Cloud PlatformDocument33 pagesCP100 Module 2 - Getting Started With Google Cloud PlatformManjunath BheemappaNo ratings yet
- Questions: Comma PlacementDocument8 pagesQuestions: Comma PlacementZarbibi Hussain khelNo ratings yet
- Making Things: The Essence and Evolution of The Toyota Production SystemDocument2 pagesMaking Things: The Essence and Evolution of The Toyota Production Systemkt44974085No ratings yet
- Pacific Plate Movement WorksheetDocument3 pagesPacific Plate Movement WorksheetJohn OsborneNo ratings yet
- A Brief About Chandrayaan 1Document3 pagesA Brief About Chandrayaan 1DebasisBarikNo ratings yet
- Ad For Natbps WB PDFDocument7 pagesAd For Natbps WB PDFrajpal singhNo ratings yet
- Philippines Research PaperDocument6 pagesPhilippines Research Paperhxmchprhf100% (1)
- Seismic Earth Pressures On Retaining WallsDocument71 pagesSeismic Earth Pressures On Retaining Wallsradespino1No ratings yet
- Upper Int U2 OnlineReviewsAndRecommendations PDFDocument2 pagesUpper Int U2 OnlineReviewsAndRecommendations PDFZsuzsa StefánNo ratings yet
- Ten Strategies For The Top ManagementDocument19 pagesTen Strategies For The Top ManagementAQuh C Jhane67% (3)
- Water TableDocument5 pagesWater TableJay DoshiNo ratings yet
- Android TV Media Player User ManualDocument17 pagesAndroid TV Media Player User ManualAneez Ahmed NNo ratings yet
- Message Staging and Logging Options in Advanced Adapter Engine of PI - PO 7.3x - 7.4 - SAP Blogs PDFDocument26 pagesMessage Staging and Logging Options in Advanced Adapter Engine of PI - PO 7.3x - 7.4 - SAP Blogs PDFSujith KumarNo ratings yet
- Structural Dynamics: 10/11/2017 Dynamic Analysis 1Document110 pagesStructural Dynamics: 10/11/2017 Dynamic Analysis 1Mohammed Essam ShatnawiNo ratings yet
- IFEM Ch07 PDFDocument19 pagesIFEM Ch07 PDFNitzOONo ratings yet