Professional Documents
Culture Documents
3'-Fam CPG
Uploaded by
AlleleBiotechOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
3'-Fam CPG
Uploaded by
AlleleBiotechCopyright:
Available Formats
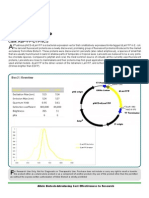
3’ FAM, 3’-FAM CPG
AHP-CH-FAM0103 100 mg
AHP-CH-FAM1003 1000 mg Deprotection: Pretreat with diethylamine or DBU for 30
minutes at room temperature. Then use ammonium hydroxide
Name: 3’-FAM CPG to cleave the oligonucleotide. This requires 2 hours at room
Chemical Name: 1-Dimethoxytrityloxy-3-[O-(N-carboxy-(3’,6’- temperature. Use an appropriate protocol (depending on the
dipivaloylfluoresceinyl)-3-aminopropyl)]-propyl-2-O-succinoyl- nucleobases) to complete the deprotection.
long chain alkylamino-CPG
Formula: N/A Storage: Freezer storage, -10 to -30°C, dry, dark area
Labeled Oligo Storage: Store labeled oligo in the dark, either
dry or in a neutral aqueous media at -20°C. Do not store crude
fluorescently labeled oligonucleotides using ammonia.
MSDS: www.allelebiotech.com
Reference:
Browne KA. Sequence-specific, self-reporting hairpin inversion probes.
J Am Chem Soc, 127, 1989. (2005)
Kim SJ, Bang EK, Kwon HJ, Shim JS, Kim BH. Modified oligonucleo-
tides containing lithocholic acid in their backbones: their enhanced cel-
lular uptake and their mimicking of hairpin structures. Chembiochem,
Use: 3’-FAM CPG is used to label fluorescein at the 3’ end of 5, 1517. (2004)
oligonucleotides.
Purity: 95%+ Dobson N, McDowell DG, French DJ, Brown LJ, Mellor JM, Brown T.
Diluent: N/A, Stability in Solution: N/A Synthesis ofHyBeacons and dual-labelled probes containing 2’-fluores-
cent groups for use in geneticanalysis. Chem Commun (Camb), 1234.
Background: (2003)
Dye-labeled oligonucleotides have many important biochemi- Kutyavin IV, Lokhov SG, Afonina IA, Dempcy R, Gall AA, Gorn VV,
cal, diagnostic, and analytical uses. Depending on the fluores- Lukhtanov E, Metcalf M, Mills A, Reed MW, Sanders S, Shishkina I, Ver-
cent properties of the dye, singly labeled oligonucleotides can meulen NM. Reduced aggregation and improved specificity of G-rich
be used for DNA sequencing and in situ hybridization. Doubly oligodeoxyribonucleotides containing pyrazolo[3,4-d]pyrimidine guanine
labeled oligonucleotides can be used as diagnostic probes bases. Nucleic Acids Res, 30, 4952. (2002)
for DNA and RNA quantification and to discriminate single
nucleotide variations (i.e., Fluorophore-Quencher, Molecular Hill KW, Taunton-Rigby J, Carter JD, Kropp E, Vagle K, Pieken W,
Beacon™). In dual labeled oligonucleotides and peptides, one McGee DP, Husar GM, Leuck M, Anziano DJ, Sebesta DP. Diels--Alder
dye acts as a fluorophore while the other acts as a quencher. bioconjugation of dienemodified oligonucleotides. J Org Chem, 66,
5352. (2001)
Fluorescein is a fluorophore. When a dual-labeled probe con-
taining fluorescein is inactive, the light emitted by the fluores- Wu H, Skrzypczynski Z, Cornwell MJ, Aboleneen H. Identification of
cein is absorbed by a neighboring quencher molecule through unexpected modifications of fluorescein-labeled oligodeoxynucleotides
Fluorescent Resonance Energy Transfer (FRET). FRET by nuclease P1 digestion and mass spectrometric techniques. Rapid
efficiency is dependent on various interactions: (1) the close Commun Mass Spectrom, 14, 26. (2000)
proximity between the excited state of the donor and accep-
tor dye molecules, (2) spectral overlap of the donor emission Hakala H, Virta P, Salo H, Lonnberg H. Simultaneous detection of sev-
spectrum with the acceptor absorption spectrum, and (3) rela- eral oligonucleotides by time-resolved fluorometry: the use of a mixture
tive orientation of the donor and acceptor dipole moments. of categorized microparticles in a sandwich type mixed-phase hybridiza-
tion assay. Nucleic Acids Res, 26, 5581. (1998)
Labeling the 3’-end of an oligo with fluorescein can be
achieved using 3’-FAM CPGs. Deprotection
���������������������������
of the result- Ramirez-Vick JE, Garcia AA, Lee J. Recovery of an oligonucleotide us-
ing oligo can be performed in the usual way with ammonium ing silver ions immobilized onto paramagnetic particles. Prep Biochem
hydroxide and will require two hours at room temperature. Biotechnol, 28, 243. (1998)
Fluorescence occurs when the fluorophore and quencher
separate—through breakage of the H-bonds that keep them Meyer KL, Hanna MM. Synthesis and characterization of a new 5-thiol-
juxtaposed. For Molecular Beacons™, this occurs when the protected deoxyuridine phosphoramidite for site-specific modification of
Molecular Beacon™ probe is hybridized to its target sequence. DNA. Bioconjug Chem, 7, 401. (1996)
Coupling: Proceed in a manner identical to the use of normal Hagmar P, Bailey M, Tong G, Haralambidis J, Sawyer WH, Davidson
nucleoside support since it contains the DMT group. BE. Synthesis and characterisation of fluorescent oligonucleotides.
Effect of internal labeling on protein recognition. Biochim Biophys Acta,
1244, 259. (1995)
You might also like
- Neural Induction FactorsDocument2 pagesNeural Induction FactorsAlleleBiotechNo ratings yet
- High Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionDocument1 pageHigh Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionAlleleBiotechNo ratings yet
- M13KO7 Helper Phage for phage displayDocument1 pageM13KO7 Helper Phage for phage displayAlleleBiotechNo ratings yet
- Luciferase PCR Template For IVTDocument1 pageLuciferase PCR Template For IVTAlleleBiotechNo ratings yet
- Modified UTPDocument1 pageModified UTPAlleleBiotechNo ratings yet
- PNCS dLanYFPDocument1 pagePNCS dLanYFPAlleleBiotechNo ratings yet
- Modified CTPDocument1 pageModified CTPAlleleBiotechNo ratings yet
- High Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionDocument1 pageHigh Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionAlleleBiotechNo ratings yet
- Cre Recombinase IVT PCR TemplateDocument1 pageCre Recombinase IVT PCR TemplateAlleleBiotechNo ratings yet
- Anti-RFP (3F5) Monoclonal AntibodyDocument1 pageAnti-RFP (3F5) Monoclonal AntibodyAlleleBiotechNo ratings yet
- ARCADocument1 pageARCAAlleleBiotechNo ratings yet
- RNA Purification SystemDocument3 pagesRNA Purification SystemAlleleBiotechNo ratings yet
- Luciferase PCR Template For IVTDocument1 pageLuciferase PCR Template For IVTAlleleBiotechNo ratings yet
- Omni-Tube PCRDocument3 pagesOmni-Tube PCRAlleleBiotechNo ratings yet
- Custom Sub Cloning ServiceDocument1 pageCustom Sub Cloning ServiceAlleleBiotechNo ratings yet
- T7 RNA PolymeraseDocument1 pageT7 RNA PolymeraseAlleleBiotechNo ratings yet
- Anti-RFP (5F8) Monoclonal AntibodyDocument1 pageAnti-RFP (5F8) Monoclonal AntibodyAlleleBiotechNo ratings yet
- Surface Bind RNA Purification SystemDocument3 pagesSurface Bind RNA Purification SystemAlleleBiotechNo ratings yet
- Sapphire Insect Transfection ReagentDocument1 pageSapphire Insect Transfection ReagentAlleleBiotechNo ratings yet
- RNA Purification SystemDocument3 pagesRNA Purification SystemAlleleBiotechNo ratings yet
- Mwasabi PCR Template For IVTDocument1 pageMwasabi PCR Template For IVTAlleleBiotechNo ratings yet
- iPS Induction IVT TemplatesDocument2 pagesiPS Induction IVT TemplatesAlleleBiotechNo ratings yet
- mClavGR2 Fusion VectorsDocument3 pagesmClavGR2 Fusion VectorsAlleleBiotechNo ratings yet
- Allele AgaroseDocument1 pageAllele AgaroseAlleleBiotechNo ratings yet
- ChromoTek GFP-multiTrapDocument3 pagesChromoTek GFP-multiTrapAlleleBiotechNo ratings yet
- ChromoTek RFP BoosterDocument1 pageChromoTek RFP BoosterAlleleBiotechNo ratings yet
- AvantGene Transfection KitDocument2 pagesAvantGene Transfection KitAlleleBiotechNo ratings yet
- pmTFP1 ClathrinDocument4 pagespmTFP1 ClathrinAlleleBiotechNo ratings yet
- Rex1-Oct3/4-Nanog iPS Methylation Detection PrimersDocument1 pageRex1-Oct3/4-Nanog iPS Methylation Detection PrimersAlleleBiotechNo ratings yet
- High Titer Luc Lentiviral ParticlesDocument1 pageHigh Titer Luc Lentiviral ParticlesAlleleBiotechNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5782)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Dosage CompensationDocument20 pagesDosage Compensationstevensb055100% (6)
- Flashcards - 1b Variety of Living Organisms - Edexcel Biology IGCSEDocument23 pagesFlashcards - 1b Variety of Living Organisms - Edexcel Biology IGCSEsohaila ibrahimNo ratings yet
- Avastin blocks VEGF to inhibit tumor growthDocument7 pagesAvastin blocks VEGF to inhibit tumor growthLoofy AnaNo ratings yet
- BIO 315. NotesDocument42 pagesBIO 315. NotesKevin KipropNo ratings yet
- Past, Present and Future of Food BiotechnologyDocument2 pagesPast, Present and Future of Food BiotechnologyJUAN C. OVIEDO LOPERANo ratings yet
- S.L. Kuznetsov, T.V. Boronikhina, V.L. GoryachkinaDocument106 pagesS.L. Kuznetsov, T.V. Boronikhina, V.L. GoryachkinaMustafa MohamedNo ratings yet
- Claribel Ria MaeDocument19 pagesClaribel Ria MaeGLENN MENDOZANo ratings yet
- PCR Overview GoldBioDocument1 pagePCR Overview GoldBioKIARAH EUNIZE GICANo ratings yet
- DNA (Deoxyribonucleic Acid)Document5 pagesDNA (Deoxyribonucleic Acid)Sagar MohanNo ratings yet
- The Krebs Cycle: Central Role in Cellular Energy ProductionDocument21 pagesThe Krebs Cycle: Central Role in Cellular Energy ProductionMusonda MulengaNo ratings yet
- Synoptic Essay PlansDocument21 pagesSynoptic Essay PlansBio_Joe50% (2)
- Cell-The Unit of LifeDocument15 pagesCell-The Unit of LifeSiddhivinayak TidakeNo ratings yet
- CRISPR/Cas genome editing of human 3PN embryosDocument2 pagesCRISPR/Cas genome editing of human 3PN embryosMaría Clara BoteroNo ratings yet
- The stage where the kinetochore microtubules of the spindle fibers separate and move the sister chromatids toward opposite poles is called AnaphaseDocument59 pagesThe stage where the kinetochore microtubules of the spindle fibers separate and move the sister chromatids toward opposite poles is called AnaphaseJames PalomariaNo ratings yet
- Cell Anatomy WorksheetDocument1 pageCell Anatomy WorksheetJayson LumagasNo ratings yet
- BIO F4 Chapter 4 Obj QuesttionDocument3 pagesBIO F4 Chapter 4 Obj QuesttionIda KamalNo ratings yet
- Meiosis: A. First Meiotic Division (Reductional Division)Document4 pagesMeiosis: A. First Meiotic Division (Reductional Division)Abbas TalibNo ratings yet
- Daku SurajDocument60 pagesDaku SurajDeepak KesharwaniNo ratings yet
- 3D Plant/Animal Cell ProjectDocument2 pages3D Plant/Animal Cell ProjectRem Rem Lai0% (1)
- Nucleic Acids As Genetic Material PDFDocument6 pagesNucleic Acids As Genetic Material PDFmanoj_rkl_07No ratings yet
- Cells and Organelles New - Answer KEY - FinalDocument9 pagesCells and Organelles New - Answer KEY - FinalMelyza Ann Pronebo50% (2)
- Biochemistry Module 7 & 8Document2 pagesBiochemistry Module 7 & 8Tricia Ann A. SodsodNo ratings yet
- 2023 All Biology Is Cognitive Information ProcessingDocument15 pages2023 All Biology Is Cognitive Information ProcessingettenmollNo ratings yet
- Dna Sequencing MethodsDocument29 pagesDna Sequencing MethodsWilson Anandaraj92% (13)
- PCR: A Powerful Technique for DNA AmplificationDocument16 pagesPCR: A Powerful Technique for DNA AmplificationGENESIS sHINENo ratings yet
- Gel Electrophoresis Basics Worksheet: NameDocument2 pagesGel Electrophoresis Basics Worksheet: Nameapi-523306558100% (1)
- Classification of EnzymesDocument7 pagesClassification of EnzymesAbhik ShrivastavaNo ratings yet
- GenbioDocument5 pagesGenbioYkhay ElfanteNo ratings yet
- Validation Test No. 1 in Grade 10 Science Quarter 3 Week 1-4Document2 pagesValidation Test No. 1 in Grade 10 Science Quarter 3 Week 1-4orlan sisonNo ratings yet
- JetQuick Plasmid Miniprep - GenomedDocument4 pagesJetQuick Plasmid Miniprep - GenomedCarlos de PazNo ratings yet