Professional Documents
Culture Documents
FTIR Acetic Acid Data
Uploaded by
arunmethaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
FTIR Acetic Acid Data
Uploaded by
arunmethaCopyright:
Available Formats
Vol.2, No.
2 (2009), 307-311
ISSN: 0974-1496
CODEN: RJCABP
http://www.rasayanjournal.com
VIBRATIONAL SPECTRA AND NORMAL COORDINATE
ANALYSIS OF ACETIC ACID CYCLOHEXYL ESTER
P. Mani1* and S. Suresh2
1
Department of Physics, Hindustan University, Padur- 603 103, India
2
Department of Physics, Loyola College, Chennai – 600 034, India
*E-mail: mani_hce@yahoo.co.in
ABSTRACT
FTIR and FTR spectra of acetic acid cyclohexyl ester have been recorded in the regions 200 - 4000 cm-1 and
30 - 4000 cm-1. The vibrational analysis has been carried out by assuming Cs symmetry. The observed
frequencies were assigned to various modes of vibrations on the basis of intensity, frequencies from allied
molecules and normal coordinate calculations. The potential energy distribution associated with normal
modes are also reported here. The assignment of fundamental vibrational frequencies for acetic acid
cyclohexyl ester agree with the calculated frequencies.
Keywords: Vibrational spectra; Normal coordinate calculation; Acetic acid cyclohexyl ester.
INTRODUCTION
Acetic acid cyclohexyl ester, derivative of acetic acid, is a colourless liquid with characteristic odour.
It has boiling point 177oC. It is immiscible in water. It is soluble in alcohol but insoluble in water and
combustible. Besides, it reacts with strong oxidants causing fire and explosion hazard. Acetic acid
cyclohexyl esters are used in large quantities as solvents for plastics, lacquers, resins and gums. It is
used as solvent for nitrocellulose, cellulose ether, bitumens, metallic soaps, basic dyes, blown oils,
crude rubber, many natural and synthetic resins and, gums and lacquers. The X-ray structures proton
and carbon-13 NMR spectra have been obtained by Baldwin and others1 for two dicyclohexyl esters and
one tricyclohexyl esters. Cyclohexyl azide is synthesized and the vibrational spectrum is recorded in
several phases including liquid at various temperatures, amorphous and crystalline at 90K2. Senyavin and
others3 reported the vibrational spectra, structure and force fields of perfluorinated cyclo and
bicycloalkanes.However, there is no report about the vibrational spectra and analysis of acetic acid
cyclohexyl ester in the literature. Hence, an attempt has been made in the present work to record the FTIR
and FTR spectra of acetic acid cyclohexyl ester and to study the complete vibrational analysis for the first
time.
EXPERIMENTAL
FTIR spectra of acetic acid cyclohexyl ester were recorded on Brucker IFS 66V FTIR spectrometer in the
region 4000 - 200 cm-1. FT Raman spectra of the same compound were also recorded on the same
instrument with FRA 106 Raman module equipped with Nd:YAG laser source operating at 1.06 µm line
with a scanning speed of 30 cm-1 min-1 of spectral width 20 cm-1. The frequencies for all sharp bands
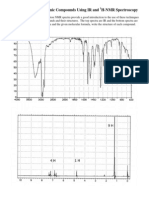
were accurate to + 1 cm-1. The molecular structure of this compound is given in Figure 1. The recorded
spectrum of acetic acid cyclohexyl ester is shown in Figure 2.
Theoretical considerations:
The geometrical symmetry possessed by the molecule helps to determine and classify the actual number
of fundamental vibrations of the system. The observed spectrum is explained on the basis of Cs point
group symmetry. The 66 fundamental vibrations are distributed as Γvib = 46a′ + 20 a". All the modes are
active in both Raman and infrared. Assignments have been made on the basis of relative intensities,
magnitudes of the frequencies and polarization of the Raman lines. The vibrational assignments are
discussed in terms of the potential energy distribution which are obtained from the evaluated potential
constants.
ACETIC ACID CYCLOHEXYL ESTERP. Mani and S. Suresh
Vol.2, No.2 (2009), 307-311
Normal coordinate Analysis:
With the modified computer program developed in this laboratory on the basis of Fuhrer et al., program4,
normal co-ordinate analysis were carried out using Wilson's
F-G matrix method. The simple general valence force field was adopted for both in plane and out of plane
vibrations. The structural parameters are taken from related molecules and Sutton's table5. The initial set
of force constants were refined by keeping a few interaction constants fixed throughout the refinement
process. The assignment to all the in plane and out of plane fundamentals are made on the basis of
intensities of Raman and IR bands, normal coordinate analysis and on comparison with those of similar
molecules.
Potential Energy Distribution:
A normalised potential energy distribution can be expressed as
where Fii are the force constants defined by damped least square technique, Lik the normalized amplitude
of the associated element (i,k) and λk the eigen value corresponding to the vibrational frequency of the
element k.The PED contribution corresponding to each of the observed frequencies over 10% are alone
listed in the present work.
RESULTS AND DISCUSSION
The observed frequencies along with their relative intensities of acetic acid cyclohexyl ester and
probable assignments are presented in table. The assignment of frequencies is made as follows.
Stretching vibrations:
C-H stretching:
The infrared bands at 3010 cm-1 and 2944 cm-1 have been assigned to C-H asymmetric stretching in CH3.
The C-H symmetric vibration in CH3 is assigned to Raman band at 2931 cm-1. The PED obtained for this
CH symmetric stretching mode in CH3 shows that it is pure mode with 94% is contributed by symmetric
C-H vibration. This molecule gives rise to eleven C-H stretching modes which are assigned to 2677,
2688, 2700, 2712, 2756, 2788, 2800, 2844, 2855, 2868 and 2905 cm-1. As expected the PED shows that
these CH stretching modes are dominated by pure stretching characters except at 2855 cm-1 and 2712 cm-
1
where there are little contributions from CC stretching along with C-H stretching. These values are good
agreement with the calculated frequencies and literature values.
C = O and C - O Stretching:
All esters have two strong characteristic bands one due to the C = O stretching vibration and the other due
to the C-O stretching vibration6. The C = O and C-O stretching modes are assigned at infrared band at
1738 cm-1 and at 1538 cm-1 which agree with calculated values.
C - C Stretching:
The six C - C stretching vibrations of the acetic acid cyclohexyl ester give rise to six absorption bands,
which can be assigned to 1050, 1075, 1100, 1119, 1145 and 1156 cm-1. The PED calculation shows that
the calculated frequencies at 1069 cm-1 and 1041 cm-1 of C-C stretchings are not pure mode whereas they
are in a mixed mode. These are combinations of C - C stretching and C - H stretching modes.
In plane and out of plane bendings:
CH – bending:
The bands observed at 1195, 1243, 1256, 1269, 1300, 1315, 1331, 1343, 1359, 1370 and 1381 cm-1 are
assigned to C-H in plane bendings while the bands observed at 650, 800, 818, 838, 863, 900, 913, 925,
943, 956 and 968 cm-1 are assigned to C - H out of plane banding vibrations. These frequencies agree
quite with the calculated values as listed in the Table 1.
CCC in plane and out of plane bendings:
Three CCC in plane bending vibrations are assigned at 605, 625 and 720 cm-1 while CCC out of plane
bending vibrations are assigned at 356, 343 and 300 cm-1. These are good agreement with calculated
frequencies.
CH3 deformation and rocking vibration:
The strong infrared band at 1456 cm-1 and very strong Raman band at 1438 cm-1 have been assigned to
CH3 deformation and CH3 rocking. The weak infrared at 438 cm-1 and Raman band at 388 cm-1 have been
assigned to CH3 wagging mode and CH3 torsion.
ACETIC ACID CYCLOHEXYL ESTERP. 308 Mani and S. Suresh
Vol.2, No.2 (2009), 307-311
CCC ring breathing and trigonal bending:
The skeleton of the molecule also gives some characteristic absorption wave number such as ring
breathing and CCC trigonal bending. In the present case, these are assigned at 856 cm-1 and 1019 cm-1
which agree quite well with literature values7-9.
CONCLUSION
A complete vibrational spectra and analysis is reported in the present work for the first time for acetic
acid cyclohexylester. The close agreement between the observed and calculated frequencies confirms the
validity of the present assignment.
REFERENCES
1. C.R. Baldwin, M.M. Britton, S.C. Davies, D.G. Gillies, D.L. Hughes, G.W. Smith and L.H.
Sutcliffe, J. Mol. Str. 403, 1, (1997).
2. D. Sulzle, A. Gatial, A.Karlsson, P.Klaeboe and C.J.Nielson, J. Mol. Str. 174, 207, (1988).
3. V.M. Senyavin, I.V. Kochikov and G.M. Kuramshina, J. Mol. Str. 410, 463, (1997).
4. H. Fuhrer, V.B. Karther, K.L. Kidel, P.J. Krugdel and H.H. Manstch Computer program for infrared
and spectrometry, normal coordinate analysis, 5, National Research Council, Ottowa, Canada
(1976).
5. L.E.Sutton, The interatomic bond distances and bond angles in molecules and ions, London Chem,
Soc London (1983).
6. D. Steele and A.Muller, J. Phys. Chem. 95, 6163, (1991).
7. S.Mohan, A.R. Prabakaran and S. Prameela, Indian J. Pure and App. Phy 29, 672, (1991)
8. George Socrates Infrared and Raman Characteristic group frequencies table & Charts Third edition
John Wiley & Sons Ltd (2001).
9. Varsangi, Assignments for vibrational spectra of seven hundred benzenz derivates, 7, Adam Hilger
London (1974)
Table-1: Observed, calculated frequencies (cm-1), vibrational assignments and potential energy
distribution of Acetic acid cyclohexyl ester
Observed frequency /
Calculated
Species Intensity Assignment % PED
wavenumber
Infrared Raman
a′ 3010 w - 3006 H asymmetric stretching in CH3 87 νasCH
a′ 2944 vs - 2942 H asymmetric stretching in CH3 92 νasCH
a′ - 2931 vw 2929 CH symmetric stretching in CH3 94 νsyCH
a′ - 2905 m 2901 C-H stretching 84 νCH
a′ 2868 vs - 2859 C-H stretching 81 νCH
a′ - 2855 m 2850 C-H stretching 79 νCH + 12 νCC
a′ 2844 vs - 2839 C-H stretching 86 νCH
a′ 2800 vw - 2804 C-H stretching 88 νCH
a′ - 2788 vw 2779 C-H stretching 74 νCH
a′ - 2756 vw 2751 C-H stretching 83 νCH
a′ - 2712 w 2108 C-H stretching 72 νCH + 17 νCH
a′ 2700 vw - 2694 C-H stretching 84 νCH
a′ - 2688 w 2681 C-H stretching 81 νCH
a′ - 2677 w 2671 C-H stretching 88 νCH
- 2113 vw - - 1100 + 1025 -
a′ 1738 vs 1732 m 1731 C = O stretching 86 νC = O
a′ 1538 w - 1530 C - O stretching 89 νC = O
a′ 1467 s 1468 w 1462 O - C stretching 71 νC = O + 19 νC - CH3
a′ 1456 v - 1451 CH3 deformation 74 δCH3 + 24 ρCH3
a′ - 1438 vs 1429 CH3 rocking 72 ρCH3 + 26 δCH3
ACETIC ACID CYCLOHEXYL ESTERP. 309 Mani and S. Suresh
Vol.2, No.2 (2009), 307-311
a′ 1381 vs - 1378 C-H in plane bending 88 βCH
a′ - 1370 w 1361 C-H in plane bending 92 βCH
a′ 1359 s - 1352 C-H in plane bending 84 βCH
a′ - 1343 w 1340 C-H in plane bending 87 βCH
a′ 1331 m 1331 w 1324 C-H in plane bending 70 βCH + 24 βCC
a′′ 1315 m - 1306 C-H in plane bending 81 βCH
a′ - 1300 w 1304 C-H in plane bending 84 βCH
a′ - 1269 w 1260 C-H in plane bending 69 βCH + 26 βCC
a′ - 1256 m 1248 C-H in plane bending 80 βCH
a′ - 1243 m 1239 C-H in plane bending 72 βCH + 24 βCO
a′ - 1195 w 1189 C-H in plane bending 79 βCH
a′ 1156 m - 1151 C-C stretching 79 νCC
a′ - 1145 m 1138 C-C stretching 86 νCC
a′ 1119 vs 1119 vw 1111 C-C stretching 82 νCC
a′ 1100 m 1100 vw 1091 C-C stretching 81 νCC
a′ - 1075 vw 1069 C-C stretching 69 νCC + 20 νCH
a′ - 1050 vw 1041 C-C stretching 74 νCC + 18 νCH
a′ 1039 vw - 1030 C-CH3 stretching 58 νC-CH3 + 26 νC=O +
14 νC-O
a′ 1019 s 1019s 1010 CCC trigonal bending 88 βCCC
a′′ 968 vs - 959 C-H out of plane bending 81 ηCH
a′′ 956 s - 950 C-H out of plane bending 79 ηCH
a′′ 943 s - 936 C-H out of plane bending 86 ηCH
a′′ - 925 vw 919 C-H out of plane bending 66 ηCH + 28 ηCC
a′′ 913 m - 907 C-H out of plane bending 81 ηCH
a′′ 900 s 900 w 894 C-H out of plane bending 69 ηCH + 21 ηCC
a′′ 863 w - 858 C-H out of plane bending 74 ηCH
a′ - 856 v 850 CCC ring breathing 91 βCCC
a′′ 838 m 838 w 828 C-H out of plane bending 79 ηCH
a′′ 818 w - 809 C-H out of plane bending 68 ηCH + 30 ηCC
a′′ 800 vw 800 vs 790 C-H out of plane bending 74 ηCH + 16 ηCC
a′ 750 vw - 739 C=O in plane bending 86 βC=O
a′ 720 vw - 709 C-O in plane bending 69 βC-O + 12 βC=O
a′ 720 vw - - CCC in plane bending
a′′ 650 m 650 mw 646 C-H out of plane bending 74 ηCH
a′ 625 vw 619 m 615 O-C in plane bending 74 βC-O + 12 βC=O
a′ 625 vw 619 m CCC in plane bending
a′ 605 m - 600 CCC in plane bending 81 βCCC
a′′ 550 w 550 m 541 C=O out of plane bending 76 ηC=O + 11 ηC-O
a′′ 481 vw - 470 C-O out of plane bending 62 ηC-O + 22 ηC=O
a′ 456 vw 456 vw 448 C-CH3 in plane bending 54 βC-CH3 + 18 βC=O +
11 βC-O
a′′ 438 w 438 w 430 CH3 wagging 66 ωCH3 + 30 τCH3
a′′ 388 vw 388 vw 378 CH3 torsion 69 τCH3 + 29 ωCH3
a′′ 356 w 350 w 331 CCC out of plane bending 74 ηCCC
a′′ - 343 w 330 CCC out of plane bending 69 ηCCC + 10 ηCH
a′′ 300 vs 300 vs 288 CCC out of plane bending 61 ηCCC + 20 ηCH
a′′ - 281 vw 271 O-C out of plane bending 64 ηC-O + 26 ηC=O +
10 ηC-CH3
a′′ - 238 vw 221 C-CH3 out of plane bending 52 ηC-CH3 + 30 ηC=O +
12 ηC-O
a′ - in-plane vibrations, a′′ - out-of plane vibrations.
ACETIC ACID CYCLOHEXYL ESTERP. 310 Mani and S. Suresh
Vol.2, No.2 (2009), 307-311
Abbreviations used: w - weak; m - medium; s - strong; vw - very weak; vs - very strong.
(Received: 24 March 2009 Accepted: 3 April 2009 RJC-354)
A scientific truth does not triumph by convincing its opponents and
making them see the light, but rather because its opponents eventually
die and a new generation grows up that is familiar with it.
-Max Planck
ACETIC ACID CYCLOHEXYL ESTERP. 311 Mani and S. Suresh
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- (Plastics Design Library) Carlos Federico Jasso-Gastinel, José M. Kenny - Modification of Polymer Properties-William Andrew (2017)Document222 pages(Plastics Design Library) Carlos Federico Jasso-Gastinel, José M. Kenny - Modification of Polymer Properties-William Andrew (2017)Monique BarretoNo ratings yet
- Geology IN - The Petroleum System PDFDocument3 pagesGeology IN - The Petroleum System PDFpranowo_ibnuNo ratings yet
- CrystalDocument8 pagesCrystalfamily_jvcNo ratings yet
- List of Aroma ChemicalsDocument4 pagesList of Aroma ChemicalsMedo MohtasebNo ratings yet
- Modern Instrumentation and Control For Nuclear Power Plants A GuidebookDocument648 pagesModern Instrumentation and Control For Nuclear Power Plants A GuidebookMark HaizlipNo ratings yet
- INCOMPATIBILITY CAUSESDocument40 pagesINCOMPATIBILITY CAUSESnizam_ghaniNo ratings yet
- Unit-Iv: Real Time Operating SystemDocument55 pagesUnit-Iv: Real Time Operating SystemarunmethaNo ratings yet
- EcosDocument36 pagesEcosarunmethaNo ratings yet
- Tank Monitoring SystemsDocument69 pagesTank Monitoring SystemsarunmethaNo ratings yet
- Multitasking Design and Implementation Issues in Embedded SystemsDocument22 pagesMultitasking Design and Implementation Issues in Embedded SystemsarunmethaNo ratings yet
- Embedded Software Primer - Ch6Document26 pagesEmbedded Software Primer - Ch6arunmethaNo ratings yet
- Embedded System On Safety Critical For Nuclear Power Plant: Sabarisan.V, Prabhu.R, Krishnaprasad.VDocument8 pagesEmbedded System On Safety Critical For Nuclear Power Plant: Sabarisan.V, Prabhu.R, Krishnaprasad.VarunmethaNo ratings yet
- Embedded System On Safety Critical For Nuclear Power Plant: Sabarisan.V, Prabhu.R, Krishnaprasad.VDocument8 pagesEmbedded System On Safety Critical For Nuclear Power Plant: Sabarisan.V, Prabhu.R, Krishnaprasad.VarunmethaNo ratings yet
- Journal of Magnetism and Magnetic Materials: Research ArticlesDocument7 pagesJournal of Magnetism and Magnetic Materials: Research ArticlesarunmethaNo ratings yet
- Unit-Iv: Real Time Operating SystemDocument55 pagesUnit-Iv: Real Time Operating SystemarunmethaNo ratings yet
- Papers-1-Nuclear Power Plant Tutorial PDFDocument4 pagesPapers-1-Nuclear Power Plant Tutorial PDFarunmethaNo ratings yet
- 10 1039@C8CS00928G PDFDocument39 pages10 1039@C8CS00928G PDFarunmethaNo ratings yet
- Iot BookDocument1 pageIot BookarunmethaNo ratings yet
- Pic PRGM ManualDocument56 pagesPic PRGM ManualSelva Raj100% (1)
- ARM Lab ManualDocument85 pagesARM Lab ManualarunmethaNo ratings yet
- Sensors and ActuatorsDocument31 pagesSensors and ActuatorsrameshsmeNo ratings yet
- Capability, Governance and Nanotechnology Developments: A Focus On IndiaDocument4 pagesCapability, Governance and Nanotechnology Developments: A Focus On IndiaarunmethaNo ratings yet
- Pic PRGM ManualDocument56 pagesPic PRGM ManualSelva Raj100% (1)
- Seni AnyamanDocument1 pageSeni Anyamanauni dalilah100% (1)
- Winter 2012 EditDocument8 pagesWinter 2012 EditarunmethaNo ratings yet
- Top 50 Baby Names of 2011 by Meaning: Contributed by Michelle MaffeiDocument2 pagesTop 50 Baby Names of 2011 by Meaning: Contributed by Michelle MaffeiarunmethaNo ratings yet
- Challenges and Prospects of Nanopillar-Based Solar Cells: 1) Device StructuresDocument15 pagesChallenges and Prospects of Nanopillar-Based Solar Cells: 1) Device StructuresarunmethaNo ratings yet
- Focus Reviews: Silica-Coated Metal NanoparticlesDocument10 pagesFocus Reviews: Silica-Coated Metal NanoparticlesarunmethaNo ratings yet
- Core Lab Course Model Curriculam M Tech NS&TDocument3 pagesCore Lab Course Model Curriculam M Tech NS&TarunmethaNo ratings yet
- Philippine Pipe Color Coding: (NBC Amended Rules and Regulations Rule XIII Electrical andDocument3 pagesPhilippine Pipe Color Coding: (NBC Amended Rules and Regulations Rule XIII Electrical andJerome ChuaNo ratings yet
- 1H, 2,4 TriazoleDocument7 pages1H, 2,4 TriazoleStas BulgariNo ratings yet
- Fe-Assisted Hydrothermal Liquefaction of Lignocellulosic Biomass For Producing High-Grade Bio-OilDocument8 pagesFe-Assisted Hydrothermal Liquefaction of Lignocellulosic Biomass For Producing High-Grade Bio-OilAYUSH DAVENo ratings yet
- Manufacturing Restricted Substances List (MRSL)Document21 pagesManufacturing Restricted Substances List (MRSL)Sajjad AhmedNo ratings yet
- English DR Soil Sericulture PDFDocument2 pagesEnglish DR Soil Sericulture PDFChathur ChathraNo ratings yet
- IR and NMR Practice 93Document8 pagesIR and NMR Practice 93Elliot JamesNo ratings yet
- 01 35 18 - LEED RequirementsDocument9 pages01 35 18 - LEED RequirementsSteve LezamaNo ratings yet
- Judging SheetDocument1 pageJudging Sheettiny66No ratings yet
- INDUSTRIAL INTERNSHIP REPORT ON MANUFACTURING OF DYESDocument50 pagesINDUSTRIAL INTERNSHIP REPORT ON MANUFACTURING OF DYES1031 Ayush NasitNo ratings yet
- Exxon™ Butyl 365S: RubberDocument2 pagesExxon™ Butyl 365S: RubberMaram YasserNo ratings yet
- Kimling Percobaan Penentuan CLDocument8 pagesKimling Percobaan Penentuan CLRinda As EtaNo ratings yet
- Ecosystem HandoutDocument8 pagesEcosystem Handout2113071023 GERALD IMMANUELNo ratings yet
- 3 PolyamidesPPTDocument22 pages3 PolyamidesPPTApoorva MNNo ratings yet
- Partes y Piezas Lavadora Choyang CYW 501 (Nuevo)Document30 pagesPartes y Piezas Lavadora Choyang CYW 501 (Nuevo)Cristian BarraNo ratings yet
- Synthesis of Grafted Polylactic Acid and Polyhydroxyalkanoate by A Green Reactive Extrusion ProcessDocument15 pagesSynthesis of Grafted Polylactic Acid and Polyhydroxyalkanoate by A Green Reactive Extrusion ProcessIrma BrennanNo ratings yet
- 2o Examen Respuestas Problema2Document2 pages2o Examen Respuestas Problema2Emmanuel RodríguezNo ratings yet
- Get AttachmentDocument7 pagesGet AttachmentGaurav PatelNo ratings yet
- ABS & PVC DWV Fittings With Alternate Fitting Patterns: NSF InternationalDocument4 pagesABS & PVC DWV Fittings With Alternate Fitting Patterns: NSF InternationalChristian D. OrbeNo ratings yet
- Ripex ApplicationsDocument3 pagesRipex ApplicationsAle VázquezNo ratings yet
- Final One Year Pivot Neet Course Phase - 1 Schedules - Ay 2023-24Document136 pagesFinal One Year Pivot Neet Course Phase - 1 Schedules - Ay 2023-24Prajwal JoshiNo ratings yet
- Materials Exercises PDFDocument7 pagesMaterials Exercises PDFKlaudio BariNo ratings yet
- AAN CHAP 4. Evaluation of Feedstuff (Digestibility Trials) - 1Document30 pagesAAN CHAP 4. Evaluation of Feedstuff (Digestibility Trials) - 1Dame NegaroNo ratings yet
- PMF 5.0 User GuideDocument136 pagesPMF 5.0 User GuideDana HowellNo ratings yet
- A Test For Solid Phase Extracted Polychlorinated Biphenyls (PCBS) Levels in Transformer OilDocument9 pagesA Test For Solid Phase Extracted Polychlorinated Biphenyls (PCBS) Levels in Transformer OilAditya Febrian MasriNo ratings yet