Professional Documents
Culture Documents
F334 June 10 Mark Scheme
Uploaded by
ExamStuffOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
F334 June 10 Mark Scheme
Uploaded by
ExamStuffCopyright:
Available Formats
GCE
Chemistry B (Salters)
Advanced GCE F334 Chemistry of Materials
Mark Scheme for June 2010
Oxford Cambridge and RSA Examinations
OCR (Oxford Cambridge and RSA) is a leading UK awarding body, providing a wide range of qualifications to meet the needs of pupils of all ages and abilities. OCR qualifications include AS/A Levels, Diplomas, GCSEs, OCR Nationals, Functional Skills, Key Skills, Entry Level qualifications, NVQs and vocational qualifications in areas such as IT, business, languages, teaching/training, administration and secretarial skills. It is also responsible for developing new specifications to meet national requirements and the needs of students and teachers. OCR is a not-for-profit organisation; any surplus made is invested back into the establishment to help towards the development of qualifications and support which keep pace with the changing needs of todays society. This mark scheme is published as an aid to teachers and students, to indicate the requirements of the examination. It shows the basis on which marks were awarded by Examiners. It does not indicate the details of the discussions which took place at an Examiners meeting before marking commenced. All Examiners are instructed that alternative correct answers and unexpected approaches in candidates scripts must be given marks that fairly reflect the relevant knowledge and skills demonstrated. Mark schemes should be read in conjunction with the published question papers and the Report on the Examination. OCR will not enter into any discussion or correspondence in connection with this mark scheme. OCR 2010 Any enquiries about publications should be addressed to: OCR Publications PO Box 5050 Annesley NOTTINGHAM NG15 0DL Telephone: Facsimile: E-mail: 0870 770 6622 01223 552610 publications@ocr.org.uk
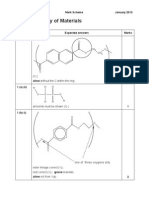
F334 Question 1 (a) alcohol / hydroxyl / hydroxy
R
Mark Scheme Expected Answers Alkene / carbon-carbon double bond / C=C Marks 2
June 2010 Additional Guidance Double bond alone does NOT score. ALLOW secondary alcohol but not primary or tertiary. Do NOT allow hydroxide. If more than 3 circles, any surplus INCORRECT ones are CON IGNORE surplus CORRECT circles 2 marks for 3 correct 2 1 mark for 2 correct 0 marks for only 1 correct
1 (b) (i)
HO
1 (b) (ii)
(The mirror images / molecules / structures / enantiomers / they) are non-superimposable / cannot be superimposed The masses of the different types of atom present are not integers / masses are measured relative to carbon-12 (12.00000) OR different compounds with the same whole number molecular mass will have different Mr values from high resolution spectra / AW AND Comparison of Mr with database / list of formulae/Mr values Peak: CH3+
1 mark independently ALLOW high resolution MS gives accurate Mr to 4 decimal places
1 (c) (i)
ALLOW calculate molecular formula by using masses of atoms involved MUST have correct charge for first mark 2 MUST be neutral for the second mark
1 (c) (ii) species lost = OH 1
F334 Question 1 (d) (i)
Expected Answers
Mark Scheme Marks
R
June 2010 Additional Guidance ALLOW this, though not full structural! DO NOT ALLOW skeletal formula
R
H H C C O
H3C C O
Mark separately 1 (d) (ii) ethanoic acid concentrated sulfuric acid / concentrated hydrochloric acid 2 IGNORE conc./ dil. / aq. for ethanoic acid Moderately is CON for acid ALLOW correct formula. e.g. CH3COOH and conc H2SO4 1 (e) C24H31NO + 2Br2 C24H31NOBr4 1 ALLOW correct formula to be given in any order of atoms
F334 Question 1 (f)
Expected Answers Any four from:
Mark Scheme Marks
June 2010 Additional Guidance Please annotate with ticks to show where ALL marks are awarded IGNORE extra points after 4 have been scored IGNORE name of solvent If no solvent is used then no marks can be scored similarly if 4. is incorrect then 5. cannot be scored
1. Heat the (impure) sample with solvent / use hot/warm solvent 2. with a minimum amount (of solvent) / add solvent to solid until just dissolves 3. filter 4. leave filtrate/solution/mixture to cool / leave to crystallise 5. filter off crystals, wash and dry AND for QWC mark: EITHER First filtration / filtration of hot solution removes insoluble impurities OR after crystallisation soluble impurities stay in solution / AW (QWC) 5
QWC only one statement required for this mark ALLOW impurities which do NOT dissolve / solid impurities ALLOW impurities which dissolve
F334 Question 1 (g)
Expected Answers
Mark Scheme Marks
June 2010 Additional Guidance Alternative answers to the 3 answers on the left: IGNORE incorrect answers, including is it toxic/harmful?, is it cost-effective? can it be modified? NOTE only one mark can be scored for each of the candidates questions (e.g. effective and better in one question only scores 1) 1. Are there any side-effects? / AW 1. What is the safe-dose? / AW
1. Is the drug safe (to be used in humans)? 3 2 Does it do the job it is designed to do? 3 Is it better than the standard treatment being used?
2. Is it effective? / does it work AW 3. Is it an improvement on other drugs? / AW 3. Can it be used to treat other symptoms/health problems / diseases? / AW ALLOW specific examples e.g. can it be used to treat cancer?
Total
21
F334 Question 2 (a) Expected Answers Fe + 2CH3COOH Fe(CH3COO)2 + H2
Mark Scheme Marks Additional Guidance Correct formula for H2 or formula of salt correct formulae AND balanced ALLOW Fe(C2H3O2)2 or Fe2+(CH3COO-)2 DO NOT ALLOW Fe(II)(CH3COO)2 Fe atom correct 2 BOTH ions correct IGNORE 4s0 for ions
June 2010
Fe atom: .... 3d6 4s2 OR 4s2 3d6 2 (b) (i) Fe(II) ion: .... 3d6 Fe(III) ion: .... 3d 2 (b) (ii) OR
5
Half-filled d-shell / Half-filled d-orbitals (more stable) 1
5
ALLOW paired electrons are less stable than if unpaired ORA
3d configuration / 3d arrangement is (more) stable (than 3d6) 2 (c) (i) Fe2+(aq) + 2OH(aq) Fe(OH)2(s) 2
ALLOW in 3d5 electrons are in separate orbitals/d-subshells Formulae correct and balanced ALLOW Fe2+(OH-)2 correct state symbols if first mark not gained: must be (aq) + (aq) (s) or (aq) + (aq) (s) + (aq) ALLOW correct formula, Fe(OH)3 / Fe2O3 / Fe2O3.xH2O ALLOW names May be shown by an equation: e.g. Fe2+ Fe3+ + eFe(OH)2 + O2 Fe2O3 IGNORE by air
2 (c) (ii)
Red-brown ppt is iron(III) hydroxide / (hydrated) iron(III) oxide Fe2+/ Fe(II) ions/Fe(OH)2 are oxidised / lose electrons 3 (by) oxygen THIS IS DEPENDENT ON Fe(II) ion/compound being oxidised
F334 2 (d) (i) 2 (d) (ii) Ce(SO4)2
Mark Scheme 1 The mark is for the working shown in bold 2
June 2010
1. moles of Ce4+ in titre = 0.100 x (18.5/1000) (= 0.00185) 2. moles of Fe2+ in 25.0 cm3 = 0.00185 moles of Fe2+ in 1000 cm3 = 0.00185 x 1000 / 25.0 = 0.0740 1. moles of A in 100 cm3 = 0.1(0) / 213 (= 4.695 x 10-4) 2 (e) (i) 2. moles of A in 1000 cm3 = 10 x 0.10 / 213 = 4.7 x 10-3 / 0.0047 2sf wavenumber / cm-1 3150 1715 bond O-H C=O location carboxylic acid ketone AND/OR carboxylic acid 1. COOH / carboxylic (acid) / carboxyl group 2 (e) (iii) (reacts with alkali) to form: 2. ions in solution / a soluble salt / salt that dissolves / soluble carboxylate (allow formula) OR 2. carboxylate/anion of carboxylic acid (allow formula) forms bonds with water 2 2
ALLOW answer to 2 sig figs i.e. 0.074 and ecf from 1 Remember that in calculations correct answer gets full marks with or without working ALLOW 4.70 x 10-3 (3 sf) BOTH bonds correct BOTH locations correct
2 (e) (ii)
IGNORE references to intermolecular bonding 2
F334 2 (f)
Mark Scheme Any 5 from the following 6 marking points but if no QWC maximum mark is 4 1. Use an appropriate/suitable filter OR a filter having the complementary colour (if named must be yellow/green) 2. (Put a sample of the reaction mixture into the colorimeter and) take absorbance readings at set (time/regular) intervals AW 3. convert absorbance readings to concentrations using the calibration curve 4. plot graph of concentration v time OR 1/time for reaction 5. determine/measure /find half-lives from graph 6. constant half-life = first order 5
June 2010 Please annotate with ticks to show marks awarded If complementary ignore any other colour QWC absorbance must be spelt correctly in either mark 2. or 3. for that mark to be allowed (this is NOT an extra mark) ALLOW absorbency but NOT absorbancy NOTE if no absorbance, max mark = 4 3. ALLOW for only one absorbance reading 4. IGNORE rate ALLOW points 5. and 6. may be shown using labelled diagrams 5. graph must refer to concentration v time plot 5. find rate of reaction by drawing tangents on graph 6. if concentration doubles and rate doubles = first order / plot of rate v [B] gives (diagonal) line (through the origin) / directly proportional
Total
24
F334 Question 3 (a) (i) Expected Answers
O C H N CH H O C
Mark Scheme Marks
June 2010 Additional Guidance ALLOW the linkage to proline ring (C,O and N atoms circled) DO NOT ALLOW if only the bond in C-N is circled
ALLOW H2NCH2COOH / full structure 1
OH
3 (a) (ii)
H2N
O
CH2
Structure must be a zwitterion to score
3 (a) (iii)
C O
-
2
+
H2N
-
ALLOW COO ALLOW + charge on H or N
COO ring structure with
NH2+
IGNORE intermolecular / any other types of intramolecular force / changing hydrogen bonds ALLOW bonds in tertiary structure IGNORE references to denaturing 2 ALLOW active site is deformed/distorted / no longer complementary/fits substrate ALLOW tertiary structure for active site
3 (b) (i)
(at high temperatures / 50C) intramolecular/hydrogen bonds break
and active site lost/altered/changed
F334 Question 3 (b) (ii)
Mark Scheme Expected Answers Marks (change of pH) affects charges on polar/some/certain groups/ active site OR ionisable groups are altered prevents correct interactions/bonds between enzyme and substrate AW Rate = k x [P] x [enzyme] 2
June 2010 Additional Guidance ALLOW a correct example (e.g. COOH, COO-, NH2, NH3+) IGNORE references to denaturing ALLOW ionic interactions/bonds are disrupted between enzyme and substrate ALLOW substrate does not fit/bind/react ALLOW hydroxylase or E or enz or complete name for enzyme ALLOW (rate equation) = k x [P] x [enzyme]; must have =
3 (c) (i) mol1 dm+3 s1 2
ALLOW units in any order and dm3 ALLOW / for -1 e.g. dm+3/ mol/ s and sec-1 ecf for units DO NOT ALLOW there are an excess of enzymes ALLOW the rate determining step is the formation of Penzyme substrate / rds involves one molecule of P
3 (c) (ii)
(When [P] is low) not all enzyme active sites are filled/saturated OR P can form a P-enzyme substrate (can be given as an equation) OR active sites available for substrates OR P can bind to active sites AW (as [P] increases) rate increases in proportion (so first order) AW
2 DO NOT ALLOW as [P] increases rate increases alone. There must be some indication of how the rate increases e.g. rate doubles as [P]/conc. of P/amount of P/number of molecules of P/P doubles ALLOW rds involves the breakdown of the enzyme-substrate complex (which does not depend on the concentration of P) 2
3 (c) (iii)
all the active sites are filled/saturated (any increase in [P] will not affect the reaction rate) OR no active site is available for P to bind to/react with (so) order becomes/is zero
F334 Question 3 (d)
Expected Answers Any two from: speeds up reaction rate reduces the number of steps in a synthesis improves the atom economy AW
Mark Scheme Marks
June 2010 Additional Guidance
ALLOW it is a one step process
reduces the amount of energy/heat required AW 2 easier separation methods enzymes can be reused/recycled uses less toxic solvents/producing less hazardous waste no/fewer organic solvents used reduces use of more toxic catalysts Total 16
ALLOW lower temperature/pressure used/needed/required
IGNORE renewed
10
F334 Question 4 (a) Expected Answers 1,4-diaminobutane
Mark Scheme Marks Additional Guidance 1,4-diamino ALLOW 1,4-diamine DO NOT ALLOW 1,4-butandiamine
June 2010
butane ALLOW butan (often in middle of name) but DO NOT ALLOW buta 1,6-diaminohexane scores 1 mark IGNORE gaps, commas and dashes extra H+ on one amino group
4 (b)
+
H3N N + H2
all correct
NH3+
ALLOW +ve charge on N or H IGNORE length of carbon chains / missing Hs on carbons ALLOW 1 mark if ALL 3 amino group Hs are correct but positive charge missing amide group correct completely correct (including carbon chains)
4 (c) (i)
N H O
H N
2 1 ACCEPT molecule the other way around. ALLOW structural formula or without brackets DO NOT ALLOW peptide DO NOT ALLOW hydrochloric acid for the first mark 2 IGNORE water formed
4 (c) (ii) 4 (c) (iii)
(Secondary) amide Hydrogen chloride / HCl a small molecule/HCl has been eliminated/formed
11
F334 Water (rather than HCl) is formed in the reaction 4 (d) HCl is toxic/harmful/dangerous/polluting (to the environment) / HCl needs to be disposed of ORA
Mark Scheme mark independently ALLOW HCl causes acid rain / is corrosive 2 second mark depends on first
June 2010
ALTERNATIVE ANSWER C contains chlorine which requires extra energy/resources to make AW Stanyl: hydrogen bond(ing) 4 (e) (i) poly(ethene): instantaneous (dipole)-induced dipole (bonds) intermolecular bonds in polythene are weaker than those in Stanyl ORA less energy / lower temperatures needed to break the imb in poly(ethene) / separate chains / enable chains to slide ORA chains can move / slide over each other (and polymer softens) Total 16 2
ALLOW id-id bonding / van der Waals forces Please annotate with ticks to show where ALL marks are awarded ALLOW intermolecular forces ALLOW for both marks named imb from 4 (e) (i), provided Stanyl bonds are stronger ALLOW less heat
4 (e) (ii)
12
F334 Question 5 (a) (i)
Expected Answers
Mark Scheme Marks
June 2010 Additional Guidance mark independently ALLOW smaller/larger since both Eo are positive IGNORE lower/higher OR references to redox processes ALLOW Eo cell for Eo, Eo for Cu(/Cu2+) is less positive than Ag(/Ag+) ALLOW from copper to silver or Cu to Ag+ IGNORE electrons flow through water / attract / gain DO NOT ALLOW from Cu2+
Since Eo for Cu(/Cu2+) is more negative than Ag(/Ag+) ORA 2 electrons will flow/move (from Cu) to Ag / from Cu/(/Cu2+ to Ag/Ag+
5 (a) (ii) 5 (a) (iii) 5 (b) (i)
0.46 V Cu + 2Ag+ Cu2+ + 2Ag Oxidising agent = H+ /H3O+ Eo values are measured with respect/compared to the (standard) H2/H+ half-cell AW / E H2/H+ = 0 metals with a negative electrode potential value will be oxidised by / will react with H+ ions/HCl AW ORA 1. Moles of Cu2+ in 250 cm3 = 0.150 x (250/1000)
1 1
ALLOW + or 0.46 IGNORE state symbols ALLOW hydrogen ions
3 ALLOW one mark for saying acids/H+ can oxidise Zn but not Cu 1. The mark is for the working shown in bold 3
5 (b) (ii) 2. Mass of copper in sample = 0.0375 x 63.5 = 2.381 3. % of Cu in brass = 2.381 / 3.97 x 100 = 60 ALLOW any number of sig. figs. ALLOW ecf from 1. and 2. DO NOT ALLOW 59 Mark separately 1 mark for each correct answer 13 13 3 ALLOW without square brackets
5 (b) (iii) formula shape coordination number Total
copper(II) complex formed with EDTA4[Cu(EDTA)] 2 octahedral 6
OCR (Oxford Cambridge and RSA Examinations) 1 Hills Road Cambridge CB1 2EU OCR Customer Contact Centre 14 19 Qualifications (General) Telephone: 01223 553998 Facsimile: 01223 552627 Email: general.qualifications@ocr.org.uk www.ocr.org.uk For staff training purposes and as part of our quality assurance programme your call may be recorded or monitored
Oxford Cambridge and RSA Examinations is a Company Limited by Guarantee Registered in England Registered Office; 1 Hills Road, Cambridge, CB1 2EU Registered Company Number: 3484466 OCR is an exempt Charity OCR (Oxford Cambridge and RSA Examinations) Head office Telephone: 01223 552552 Facsimile: 01223 552553 OCR 2010
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Minerals To Medicines Mark Scheme June 2002Document3 pagesMinerals To Medicines Mark Scheme June 2002ExamStuffNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Minerals To Medicines Mark Scheme Jan 2002Document4 pagesMinerals To Medicines Mark Scheme Jan 2002ExamStuffNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- L A Level Chemistry Salters MS Jan 06Document52 pagesL A Level Chemistry Salters MS Jan 06ExamStuffNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Minerals To Medicines Mark Scheme Jan 204Document3 pagesMinerals To Medicines Mark Scheme Jan 204ExamStuffNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- L A Level Chemistry Salters MS Jan 08Document24 pagesL A Level Chemistry Salters MS Jan 08ExamStuffNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Minerals To Medicines Mark Scheme Jan 2003Document3 pagesMinerals To Medicines Mark Scheme Jan 2003ExamStuffNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Minerals To Medicines Mark Scheme June 2003Document3 pagesMinerals To Medicines Mark Scheme June 2003ExamStuffNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- f334 Jun 10 - New SpecDocument20 pagesf334 Jun 10 - New SpecExamStuffNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- F334 Jun 09 - MSDocument8 pagesF334 Jun 09 - MSExamStuffNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- F334 Jun 08 - MSDocument6 pagesF334 Jun 08 - MSExamStuffNo ratings yet
- F334 June 10 Mark SchemeDocument16 pagesF334 June 10 Mark SchemeExamStuffNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- F334 Jun 09Document20 pagesF334 Jun 09ExamStuffNo ratings yet
- F334 Jun 10 - MSDocument7 pagesF334 Jun 10 - MSExamStuffNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- F334 - 07 Jun - QPDocument20 pagesF334 - 07 Jun - QPExamStuffNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- f334 Jan 10 - New SpecDocument20 pagesf334 Jan 10 - New SpecIbrahimAhmed1994No ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- F334 Jun 07 - MSDocument5 pagesF334 Jun 07 - MSExamStuffNo ratings yet
- F334 Jun 06Document16 pagesF334 Jun 06ExamStuffNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- F334 Jun 04Document8 pagesF334 Jun 04ExamStuffNo ratings yet
- F334 Jan 10Document20 pagesF334 Jan 10ExamStuffNo ratings yet
- F334 Jun 06 - MSDocument7 pagesF334 Jun 06 - MSExamStuffNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- F334 Jun 03Document8 pagesF334 Jun 03ExamStuffNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- F334 JAN 10 - MS (New Spec)Document13 pagesF334 JAN 10 - MS (New Spec)ExamStuffNo ratings yet
- F334 Jun 02Document8 pagesF334 Jun 02ExamStuffNo ratings yet
- F334 Jan 10 - MSDocument8 pagesF334 Jan 10 - MSExamStuffNo ratings yet
- F334 Jan 06 - MSDocument5 pagesF334 Jan 06 - MSExamStuffNo ratings yet
- F334 Jan 08 - MSDocument5 pagesF334 Jan 08 - MSExamStuffNo ratings yet
- F334 Jan 09 - MSDocument9 pagesF334 Jan 09 - MSExamStuffNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- F334 Jan 07Document16 pagesF334 Jan 07ExamStuffNo ratings yet
- F334 - 06 Jan - QPDocument20 pagesF334 - 06 Jan - QPExamStuffNo ratings yet
- 1.7 1.8 1.9 Enzyme Action and InhibitionDocument2 pages1.7 1.8 1.9 Enzyme Action and InhibitionHroaldrNo ratings yet
- L3.2 Immobilized Enzyme KineticsDocument98 pagesL3.2 Immobilized Enzyme KineticsRalph Evidente100% (2)
- Enzymes & Metabolism (2.1.4) AQA GCSE Biology Revision Notes 2018 Save My Exams 2Document1 pageEnzymes & Metabolism (2.1.4) AQA GCSE Biology Revision Notes 2018 Save My Exams 2Zakaria KhanNo ratings yet
- Service Training: Bio-Chemistry Global Tech Support DepDocument147 pagesService Training: Bio-Chemistry Global Tech Support DepĐỗ NamNo ratings yet
- Amylase Activity TestDocument14 pagesAmylase Activity TestSanya chauhanNo ratings yet
- Enzymes 1Document6 pagesEnzymes 1zarszNo ratings yet
- Unit - 3Document11 pagesUnit - 3Yitbarek TesfayeNo ratings yet
- Biology Lab 6 EnzymesDocument5 pagesBiology Lab 6 EnzymesMarc MohammedNo ratings yet
- Enzymes and Metabolism: Multiple-Choice QuestionsDocument49 pagesEnzymes and Metabolism: Multiple-Choice QuestionsRyanNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Hole's Human Anatomy and Physiology: Shier Butler LewisDocument12 pagesHole's Human Anatomy and Physiology: Shier Butler LewisTheophilus BaidooNo ratings yet
- Long Answer Questions A LEVEL BIOLOGYDocument205 pagesLong Answer Questions A LEVEL BIOLOGYakil75% (4)
- Biological Catalysts: IGCSE Biology (Cambridge)Document40 pagesBiological Catalysts: IGCSE Biology (Cambridge)송준혁No ratings yet
- Enzymes ReviewerDocument2 pagesEnzymes ReviewerAshley MalolotNo ratings yet
- Bi Substrate ReactionsDocument14 pagesBi Substrate ReactionsIda Nancy KNo ratings yet
- 9 BiomoleculesDocument24 pages9 BiomoleculesCBSE123.CO.NR100% (3)
- Enzyme Catalysis LabDocument7 pagesEnzyme Catalysis LabChristian JimenezNo ratings yet
- Chem Soc Rev: Review ArticleDocument29 pagesChem Soc Rev: Review ArticleAngélica Andrea SalinasNo ratings yet
- Questions and Mark Scheme Enzymes 02 To 08 SL N HLDocument39 pagesQuestions and Mark Scheme Enzymes 02 To 08 SL N HLmedic8209100% (1)
- BBL Crystal Identification Systems Enteric-Nonfermenter ID KitDocument16 pagesBBL Crystal Identification Systems Enteric-Nonfermenter ID KitWinona ShenaniNo ratings yet
- 2 4-EnzymesDocument77 pages2 4-EnzymesZewdu TekiluNo ratings yet
- RP - PhotosynthesisDocument29 pagesRP - PhotosynthesisNeel MutagiNo ratings yet
- AP Bio EnzymesDocument50 pagesAP Bio Enzymesjulie raines100% (6)
- Chapter 5 Metabolism and EnzymesDocument8 pagesChapter 5 Metabolism and Enzymes绿谷出久No ratings yet
- Chemical Biology PresentationDocument26 pagesChemical Biology PresentationGourav DasNo ratings yet
- Enzymes PPDocument28 pagesEnzymes PPEthan-Dale BrownNo ratings yet
- CAIE Biology A-Level: Topic 3: EnzymesDocument4 pagesCAIE Biology A-Level: Topic 3: EnzymesChinguun MunkhtsetsegNo ratings yet
- Bio Lab - Enzymes and Temp.Document2 pagesBio Lab - Enzymes and Temp.ZahraNo ratings yet
- Richard Kilgo Enzyme Controlled Reactions WorksheetDocument4 pagesRichard Kilgo Enzyme Controlled Reactions Worksheetcraigkilgo100% (5)
- Lab 13 Enzymes Reading PDFDocument8 pagesLab 13 Enzymes Reading PDFLarryDengNo ratings yet
- Edexcel International A Levels Biology Unit 3 Wbi13Document7 pagesEdexcel International A Levels Biology Unit 3 Wbi13Bara' Hammadeh100% (1)