Professional Documents
Culture Documents
Nickel
Uploaded by
Meruyert Kaldybekova0 ratings0% found this document useful (0 votes)
37 views46 pagesPure nickel is ductile and tough because it possesses a Iace-centered cubic (Icc) structure up to its melting point (1453 C. Or 2647 F) nickel and nickel alloys are readily Iabricated by conventional methods. And they oIIer Ireedom Irom the ductile-to-brittle transition behavior oI other metals and alloys. Including steels.
Original Description:
Original Title
54287893-Nickel
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentPure nickel is ductile and tough because it possesses a Iace-centered cubic (Icc) structure up to its melting point (1453 C. Or 2647 F) nickel and nickel alloys are readily Iabricated by conventional methods. And they oIIer Ireedom Irom the ductile-to-brittle transition behavior oI other metals and alloys. Including steels.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
37 views46 pagesNickel
Uploaded by
Meruyert KaldybekovaPure nickel is ductile and tough because it possesses a Iace-centered cubic (Icc) structure up to its melting point (1453 C. Or 2647 F) nickel and nickel alloys are readily Iabricated by conventional methods. And they oIIer Ireedom Irom the ductile-to-brittle transition behavior oI other metals and alloys. Including steels.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 46
Nickel and Nickel Alloys
NICKEL AND NICKEL-BASE ALLOYS are vitally important to modern
industry because oI their ability to withstand a wide variety oI severe operating
conditions involving corrosive environments. high temperatures. high stresses.
and combinations oI these Iactors. There are several reasons Ior these
capabilities. Pure nickel is ductile and tough because it possesses a Iace-centered
cubic (Icc) structure up to its melting point (1453 C. or 2647 F). ThereIore.
nickel and nickel alloys are readily Iabricated by conventional methods
Wrought. cast. and powder metallurgy (P/M) products are available --.and they
oIIer Ireedom Irom the ductile-to-brittle transition behavior oI other metals and
alloys. including steels. Nickel has good resistance to corrosion in the normal
atmosphere. in natural Ireshwaters. and in deserted no oxidizing acids. and it has
excellent resistance to corrosion by caustic alkali's. ThereIore. nickel oIIers very
useIul corrosion resistance. and it is an excellent base on which to develop
specialized alloys that can capitalize on the unique properties oI speciIic alloying
elements.
Because nickel has an extensive solid solubility Ior many alloying elements. the
microstructure oI nickel alloys consists oI the Icc solid-solution austenite (v) in
which dispersion and precipitate particles can Iorm. Nickel Iorms a complete
solid solution with copper and has nearly complete solubility with iron. It can
dissolve 35 Cr. 20 each oI molybdenum and tungsten. and 5 to 10 each
oI aluminum. titanium. manganese. and vanadium. Thus. the tough. ductile Icc
1
Matrix can dissolve extensive amounts oI elements in various combinations to
Provide solution hardening. as well as improved corrosion and oxidation
resistance. The degree oI solution hardening has been related to the atomic size
DiIIerence between nickel and the alloying element. and thereIore the ability oI
the solute to interIere with dislocation motion. Tungsten. molybdenum.
niobium. tantalum. and aluminum. when aluminum is leIt in solution. are strong
solution hardeners. with tungsten. niobium. tantalum and molybdenum also
being eIIective at temperatures above 0.6 Tm (Tm is melting temperature). where
diIIusion-controlled creep strength is important. Iron. cobalt. titanium.
chromium. and vanadium are weaker solution-hardening elements.
Finally. unique intermetallic phases can Iorm between nickel and some nickel
alloying elements. For example. aluminum and titanium are usually added
together Ior the age-hardening Y' precipitate Ni3 (Al. Ti). This enables the
Iormation oI alloys with very high strengths Ior both low-and high-temperature
services.
Mining and Extraction
Nickel Ores. There are two main types oI nickel ores; sulIide and oxide SulIide
ores are most commonly extracted Irom underground mines with recovery by
typical "hard-rock' mining methods. Open-pit mining is practiced to a much
lesser extent. Oxide ores. in contrast. are recovered Irom earthy surIace deposits
by direct excavation using conventional earth-moving methods and equipment.
SulIide ore mining is. thereIore. typiIied by higher costs relative to those
incurred Ior the oxide. However. the valuable mineral components oI the sulIide
ores are readily amenable to concentration; hence the maiority oI extracted
2
nickel originates Irom sulIide ores. Once mined and crushed. sulIide ores are
readily upgraded by Ilotation and magnetic separation allowing the separation oI
nickel. copper. and iron concentrates. Pyrometallurgical. hydrometallurgical.
electrolytic. and vapometallurgical processes are used to reIine the concentrates
to elemental metal.
Pyrometallurgy. The concentrates are calcined in a roasting Iurnace. smelted in
a reverberatory or other similar Iurnace using coal or natural gas Ior I uel. and
blown with air in a converter. Nickel and copper concentrates are Iurther reIined
to increase the purity oI the metals. Nickel is reIined to lower to an acceptable
level those elements that would have a deleterious eIIect on subsequent metal
perIormance. The elements removed include antimony. arsenic. bismuth. copper.
iron. lead. phosphorus. sulIur. tin. and zinc. The maior and minor impurities
removed are also recovered iI economically viable. Precious metals present at
minute levels in sulIide ores are ultimately recovered to complete the total
reIining/recovery process.
Hydrometallurgy. As an alternative to smelting pressure ammonia leaching is
used to treat nickel sulIide ore concentrate. aIter which reIined nickel power is
precipitated Irom the leach solution by hydrogen reduction. Atmospheric-
pressure ammonia leaching has also been used to recover iron and nickel Irom
roasted and reduced iron-rich sulIide concentrate.
Electrolytic Processes. ElectroreIined and electro winning both are electrolytic
processes used to produce pure nickel cathode sheet. In the Iormer process. a
nickel electrolytic reIining cell consisting oI a slab oI crude nickel to be reIined
(anode) and a thin nickel starting sheet (cathode) are immersed in an aqueous
electrolyte. and direct current is passed through the cell. Nickel and some
3
impurities are dissolved in the electrolyte (anolyte). The solution is pumped Irom
the cell. and the impurities are chemically removed. PuriIied electrolyte is
returned to the cell as catholyte and deposited on the cathode as pure nickel.
Electro winning. also an electrolytic process. can electrolyte soluble anodes oI
nickel sulIide or utilize insoluble anodes to extract the nickel Irom a leach liquor.
Vapometallurgical. The carbonyl process uses gas-to-metal transIormation to
extract pure nickel Irom an impure nickel oxide. In this process. the oxide is
reduced with hydrogen and the nickel reacts selectively with carbon monoxide to
Iorm gaseous nickel carbonyl. The gas is composed by heat to yield pure nickel.
This is acknowledged as the best available method oI reIining nickel. The power
or pellet produced is oI a very high purity. the process is energy eIIicient. and
there are no polluting waste by products.
Use Nickel Consumption.
Stainless steel 62.7
Nickel-base alloys 11.9
Plating 9.7
Alloy steels 9.0
Foundry products 3.5
Copper-base alloys 1.4
Other 1.8
Source: Nickel Development Institute
Stainless steels. principally the austenitic 300 series. are by Iar the largest
consumers oI nickel.
Nickel is added to stainless steels to improve Iormability. weld ability. corrosion
resistance. strength and oxidation resistance at elevated temperature. low-
temperature toughness. and strength attainable by cold work.
4
Nickel and nickel alloys are used Ior a wide variety oI applications. the maiority
oI which involve corrosion resistance and/or heat resistance. Some oI these
include components used in the chemical and petrochemical industries. pollution
control equipment. coal gasiIication and liqueIaction systems. parts used in pulp
and paper mills. aircraIt gas turbines. team turbine power plants. turbochargers
and valves in reciprocating engines. electrical and electronic applications. and
heat-treating equipment. A number oI other applications Ior nickel alloys involve
the unique physical properties oI special-purpose alloys. such as controlled-
expansion alloys. electrical-resistance alloys. soIt-magnetic alloys. and shape-
memory alloys. Compositions. mechanical properties. and the corrosion
resistance oI nickel and nickel alloys are described in the Iollowing paragraphs.
Plating is used primarily Ior decorative coatings. although industrial coatings
and electroIorming applications are signiIicant. The principal obiectives oI
nickel plating are to improve appearance. surIace Iinish. corrosion resistance.
and wear resistance.
Alloy Steels. Nickel. in amounts up to 9. is an important constituent oI many
alloy steels. It is Irequently used in coniunction with other alloying elements
(such as chromium and molybdenum) to permit development oI optimum
hardness. strength. ductility. corrosion resistance. and processing characteristics.
Nickel lowers the ductile-to brittle transition temperature oI steel. making nickel-
containing steels useIul Ior transporting and handling liqueIied gases and Ior
machinery and structures used at subzero temperatures.
5
Foundry Products. Nickel. in amounts up to 35. is an important constituent oI
heat-and corrosion-resistant cast stainless steels. It is also used in alloyed cast
irons; austenitic high-alloy gray and ductile cast irons containing 15 to 35
nickel. Engineering grades oI cast low-alloy steels also contain nickel.
Copper Alloys. The corrosion resistance. heat corrosion resistance. heat
conductivity. and strength oI copper-nickel alloys containing 10 to 30 Ni
account Ior their use in heat exchangers. particularly in sea water. Nickel is also
used in nickel silvers (Cu-Ni-Zn alloys containing up to 25 Ni) and cast
bronzes.
Other uses Ior nickel include chemicals. salts. catalysts. batteries. and other
miscellaneous applications.
Categories of Nickel and Nickel Alloys
Nickel and nickel alloys. like the stainless steels. oIIer a wide range oI corrosion
resistance. However. nickel can accommodate larger amounts oI alloying
elements. chieIly chromium. molybdenum. and tungsten. in solid solution than
iron. ThereIore. nickel-base alloys. in general. can be used in more severe
environments than the stainless steels. In Iact. because nickel is used to stabilize
the austenite (Icc) phase oI some oI the highly alloyed stainless steels. the
boundary bet wean these and nickel-base alloys is rather diIIuse. For example.
the UniIied Numbering System (UNS) Ior metals and alloys classiIies the
Iollowing austenitic stainless steels as nickel alloys:
Material UNS No.
20Cb3 (Fe-35Ni-20Cr) N08020
6
20M0-4 (Fe-37Ni-23Cr-4M0) N08024
20M0-6 (Fe-35Ni-24Cr-6M0) N08026
Al-6X (Fe-24Ni-21Cr-7M0) N08366
Table 1 Compositions wrought corrosion-resistant nickel-base alloys
Composition. wt (a)
Alloy UNS No. Ni Cu Fe Mn C Si Si Other
Commercially pure and low-alloy nickels
Nickel 200 NO2200 99.0 min 0.25 0.40 0.35 0.15 0.35 0.01 ..
Nickel 201 N02201 99.0 min 0.25 0.40 0.35 0.02 0.35 0.01 ...
Nickel 205 N02205 99.0 min (b) 0.15 0.20 0.35 0.15 0.15 0.008 0.001-0.08 Mg.0.01-005Ti
Nickel 211 N02211 93.7 min (b) 0.25 0.75 4.25-5.25 0.20 0.15 0.015 ..
Nickel 212 .. 97.0 min 0.20 0.25 1.5-2.5 0.10 0.20 .. 0.20 Mg
Nickel 222 ... 99.0 min (b) 0.10 0.10 0.30 . 0.10 0.008 0.01-0.10 Mg. 0.005 Ti
Nickel 270 N02270 99.9 min 0.01 0.05 0.003 0.02 0.005 0.003 0.005 Mg. 0.005 Ti
Nickel 290 N02290 bal 0.02 0.015 0.001 0.006 0.001 0.0008 0.001 Al. 0.001 Mg
Duranickel 301 N03301 93.00 mon 0.25 0.60 0.50 0.30 1.00 0.01 4.00-4.75 Al.
0.25-100 Tl
Nickel-copper alloys
Alloy 400 N04400 63.0 min (b) 28.0-34.0 2.5 0.20 0.3 0.5 0.024 ..
Alloy 401 N0441 40.0-45.0 (b) bal 0.75 2.25 0.10 0.25 0.015 ..
Alloy 404 N04404 52.0-57.0 bal 0.50 0.10 0.15 0.10 0.024 0.05 Al
Alloy R-405 N04405 63.0 min (b) 28.0-34.0 2.5 2.0 0.3 0.5 0.25-0.060 ...
Alloy K-500 N05500 63.0 min (b) 27.0-33.0 2.0 1.5 0.25 0.5 0.01 2.30-3.15 Al.0.35-0.85 Tl
A distinction is usually made between alloys that are primarily used Ior bight-
Composition. wt (a)
Alloy UNS No. Ni Cr Fe Co Mo W Nb Tl Al C Mn Si B Other
Nickel-chromium-iron (-molybdenum) alloy
Alloy 600 N06600 72.0 min(b) 14.0-17.0 6.0-10.0 ... ... ... .. .. .. 0.15 1.0 0.5 .. 0.5 Cu
Alloy 601 N06601 58.0-63.0 21.0-25.0 bal .. . . . .. 1.0-1.7 0.10 1.0 0.50 . 1.0Cu
Alloy 617 N06617 44.5 min 20.0-24.0 3.0 10.0-15.0 8.0-10.0 . . 0.6 0.8-1.5 0.05-0.15 1.0 1.0 0.006 0.5 Cu
Alloy 625 N06625 58.0 min 20.0-23.0 5.0 1.0 8.0-10.0 . 3.15-4.15 0.40 0.40 0.10 0.50 0.50 ... ...
Alloy 690 N06690 58.0 min 27.0-31.0 0-11.0 ... .. .. ... ... ... 0.05 0.50 0.50 .... 0.50 Cu
Alloy 718 N07718 50.0-55.0(b) 17.0-21.0 bal 1.0 2.8-3.30 .. 4.75-5.50(c) 0.65-1.15 0.20-0.80 0.08 0.35 0.35 0.006 0.30 Cu
Alloy 725 N07725 55.0-59.0 19.0-22.5 bal .. 7.0-9.5 ... 2.75-4.0 1.0-1.70 0.35 0.03 0.35 0.20 ... ....
Iron-nickel-chromium (-molybdenum) alloys
Alloy 800 N08800 30.0-35.0 19.0-23.0 39.5 min .. ... .. ... 0.15-0.60 0.15-0.60 0.10 1.5 1.0 ... ...
Alloy 800HT N08811 30.0-35.0 19.0-35.0 39.5 min .. ... ... .... 0.15-0.60 0.60-0.60 0.06-0.10 1.5 1.0 ... 0.895-1.20
Al Tl
Alloy 825 N08825 38.0-46.0 19.5-23.5 22.0 min ... 2.5-3.5 .. ... 0.6-1.2 0.2 0.05 1.0 0.5 .... .....
Alloy 925 N09925 38.0-46.0 19.5-23.5 22.0 min .... 2.5-3.0 .. 0.50 1.9-2.4 0.10-0.50 0.03 1.0 0.50 ... ...
20Cb3 N08020 32.0-38.0 19.0-21.0 bal ... 2.0-3.0 .. 1.0 ... .... 0.07 2.0 1.0 ... 3.0-4.0Cu
Nickel-chromium-molybdenum (-iron) alloys or nickel-molybdenum alloys
Alloy B NI 0001 bal 1.0 6.0 2.5 26.0-33.0 .. .. .... ... 0.12 1.0 1.0 ..... 0.60 V
Alloy B-2 NI 0665 bal 1.0 2.0 1.0 26.0-30.0 ... .... .... ... 0.02 1.0 0.10 ..... ....
Alloy B-3 Nl 0675 65.0 1.0-3.0 1.0-3.0 3.0 27.0-32.0 3.0 0.20 0.20 0.50 0.01 3.0 0.10 ..... 0.20 Ta
Alloy C Nl 0002 bal 14.5-16.50 4.0-7.0 2.5 15.0-17.0 3.0-4.5 ... .. .. 0.08 1.0 1.0 ... 0.35 V
Alloy C-4 N06455 bal 14.0-18.0 3.0 2.0 14.0-17.0 .. .. 0.70 .... 0.015 1.0 0.08 ..... ....
Alloy C-22 N06022 bal 20-0-22.5 2.0-6.0 2.5 12.5-14.5 2.5-3.5 .. .... ... 0.015 0.5 0.08 ..... 0.35 V
Alloy C-276 Nl 0276 bal 14.5-16.50 4.0-7.0 2.5 15.0-17.0 3.0-4.5 .. .... ... 0.02 1.0 0.08 ..... 0.35 V
Alloy G N06007 bal 21.0-23.5 18.0-21.0 2.5 5.5-7.5 1.0 1.75-2.5 ... ... 0.05 1.0-2.0 1.0 ..... .....
Alloy G-2 N06975 47.0-52.0 23.0-26.0 bal . 5.0-7.0 ... ... 0.70-1.5 ... 0.03 1.0 1.0 ... 0.70-1.20Cu
Alloy G-3 N06985 bal 21.0-23.5 18.0-21.0 5.0 6.0-8.0 1.5 .. ... .. 0.015 1.0 1.0 .... 1.5-2.5 Cu
0.50
NbFa
Alloy G-30 N06030 bal 28.0-31.5 13.0-17.0 5.0 4.0-6.0 1.5-4.0 0.3-1.5 ... .... 0.03 1.5 0.8 .... 1.0-2.4 Cu
Alloy N N10003 bal 6.0-8.0 5.0 0.2 15.0-18.0 0..5 .... .... .... 0.010 1.0 1.0 0.010 0.50V.0.35
Cu
Allcorr N06110 bal 27.0-33.0 .... 12.0 8.0-12.0 4.0 2.0 1.5 1.5 0.15 .. ... ... ...
(a) Single values are maximum unless otherwise indicated. (b) Nickel plus cobalt content
7
temperature strength. commonly reIerred to as super alloys. and alloys that are
primarily used Ior corrosion resistance. Again. the distinction is not sharp.
because some oI the super alloys are used in corrosion service and some oI the
corrosion-resistant alloys are used in high-temperature service. Many oI the
alloys that have high-temperature strength are multiphase alloys with
precipitation-strengthening elements such as aluminum. titanium. and niobium.
They also have higher carbon levels. Most oI the corrosion-resistant alloys are
primarily single-phase alloys that can be strengthened mainly by cold working.
Wrought Corrosion-Resistant Alloys
Wrought corrosion-resistant nickel-base alloys range in composition Irom
commercially pure nickel to complex alloys containing many alloy elements.
Table 1 lists the compositions oI commonly used alloys. Representative
mechanical properly data are given in Table 2.
The commercially pure nickel grades.
Nickel 200 to 205. are highly resistant to many corrosive media. especially in
reducing environments. but also in oxidizing environments where they can
maintain the passive nickel oxide surIace Iilm. They are used in the chemical
processing and electronics industries. They are hot worked at 650 to 1230 C
(1200 to 2250 F).annealed at 700 to 925 C (1300 to 1700 F). and are hardened
by cold working. For processed sheet. Ior example. The tensile properties in the
annealed condition (460 MPa. Or 67 ksi. tensile strength; 148 MPa. Or 22 ksi.
yield strength; and 47 elongation) can be increased by cold rolling up to 760
MPa (110 kis) tensile strength. 635 MPa (92 ksi) yield strength. and 8
8
Elongation. Because oI nominal 0.08 C content (0.15 max). Nickel 200
(UNS N02200) should not be used above 315 C (600 F). because embitters-
Meant results Irom the precipitation oI graphite in the temperature range 425 to
650 C (800 to 1200 F). The more widely used low-carbon alloy Nickel 201
(UNS N02201) . with 0.02 max C. can be used at temperatures above 290 C
(550 F). Higher-purity nickel is commercially available Ior various electrical
applications.
Low-alloy nickels contain 94 min Ni. The 5 Mn solid-solution addition in
Nickel 211 protects against sulIur in service environments. As little as 0.005 S
can cause liquid embitterment at unalloyed nickel grain boundaries in the range
between 640 and 740 C (1185 and 1365 F). Cupronickel alloy 301 (Ni-4.5Al-
0.6Ti). oIIers the corrosion resistance oI commercially pure nickel with the
strengthening provided by the precipitation oI Y'. There is suIIicient alloying
additions in alloy 301 to lower the Curie temperature. making the alloy weakly
Ierromagnetic at room temperature.
Nickel-copper alloys are strong and tough. oIIering corrosion resistance in
various environments. including brine and sulIuric and other acids. and showing
impunity to chloride-ion stress corrosion. They are used in chemical processing
and pollution control equipment. Capable oI precipitating Y' . Ni 3 (Al.Ti) with
2.7Al-0.6Ti alloy addition. alloy K-500 adds an age-hardening component to the
good solution strengthening and work-hardening characteristics already available
with the nominal 30 Cu in alloy 400.The composition oI these alloys can be
adiusted to decrease the Curie temperature to below room temperature.
The Ni-Cr-Fe(-Mo) alloys might simply be thought oI as nickel-base analogs oI
9
the iron-base austenitic stainless steel alloys. with an interchange oI the iron and
nickel contents. In these commercially important alloys the chromium content in
general ranges Irom 15 to 30. and iron ranges Irom 3 to 20 With a well-
maintained Cr2 O 3 surIace Iilm. these alloys oIIer excellent corrosion resistance
in many sever environments. showing immunity to chloride-ion stress-corrosion
cracking (SCC). They also oIIer good oxidation and sublimation resistance with
good strength at elevated temperatures. These nickel-rich Ni-Cr-Fe alloys have
maximum operating temperatures in the neighborhood oI 1200 C (2200 F).
Alloy 600 (UNS N06600. with Ni-15Cr-8Fe) is a single-phase alloy that can be
used at temperatures Irom cryogenic to 1093 C (2000 F). The modest yield
strength oI strip in the annealed condition (207 to 310 MPa. or 30 to 45 ksi) can
be readily work hardened by cold rolling to reach yield strengths oI 827 to 1100
MPa (120 to 160 ksi) and can retain most oI this strength up to 540 C
(1000 F).
The Fe-Ni-Cr(-Mo) alloys are also an extension oI stainless steel technology.
Incoloy alloy 800 was designed as a leaner nickel version oI the Ni-Cr-Fe series
oI materials described previously. It oIIered god oxidation resistance and was
introduced as a sheathing material Ior electric stove elements. The 800 alloy
series has also Iound extensive use in high-temperature petrochemical
environments. where sulIur-containing Ieedstock's (naphtha and heavy oils) are
cracked into component distillate parts. Not only are they resistant to chloride-
ion SCC. but they also oIIer resistance to polyphonic acid cracking. (Alloy 801
oIIers optimum resistance.) The Fe-Ni-Cr alloys also oIIer excellent strength at
elevated temperature (creep and stress rupture).
The Ni-Cr-Mo (-Fe) alloys consist oI a large Iamily oI alloys that are used in
10
the chemical processing. pollution control. and waste treatment industries to
utilize their excellent heat and corrosion resistance. Alloys in this commercially
important Iamily. such as C-276 and alloy 625. are made even more versatile by
their excellent welding characteristics and the corrosion resistance oI welded
structures. The molybdenum additions to these alloys improve resistance to
pitting and crevice corrosion. The high molybdenum (-28) in Hostelry B. B-2.
and B-3 promote good corrosion resistance in the presence oI hydrochloric acid
and other strongly reducing chemicals.
Table 2 Room-temperature mechanical properties of selected nickel-base alloys
Ultimate Yield strength Elongation in Elastic
Tensile strength (0.2 oIIset) 50 mm modulus (tension)
Alloy MPa Ksi MPa Ksi (2 in.). GPa 106 psi Hardness
Nickel 200 462 67 148 21.5 47 204 29.6 109 HB
Nickel 201 403 58.5 103 15 50 207 30 129 HB
Nickel 205 345 50 90 13 45 .. .. .....
Nickel 211 530 77 240 35 40 .. .... ...
Nickel 212 483 70 .. .. .. ... ... .....
Nickel 222 380 55 ... .... .. .... ......
Nickel 270 345 50 110 16 50 ..... .... 30 HRB
Duranickel 301
(precipitation hardened) 1170 170 862 125 25 207 30 30-40 HRC
Alloy 400 550 80 240 35 40 180 26 110-150 HB
Alloy 401 440 64 134 19.5 51 ... .. ...
Alloy R-405 550 80 240 35 40 180 26 110-140 HB
Alloy K-500
(precipitation hardened) 1100 160 790 115 20 180 26 300 HB
Alloy 600 655 95 310 45 40 207 30 75 HRB
Alloy 601 620 90 275 40 45 207 30 65-80 HRB
Alloy 617 (solution annealed) 755 110 350 51 58 211 30.6 173 HB
Alloy 625 930 135 517 75 42.5 207 30 190 HB
Alloy 690 725 105 348 50.5 41 211 30.6 88 HRB
Alloy 718
(Pnecipitation hardened) 1240 180 1036 150 12 211 30.6 36 HRC
Alloy C-22 785 114 375 54 62 .. .. 209 HB
Alloy C-276 790 115 355 52 61 205 29.8 90 HRB
Alloy G3 690 100 320 47 50 199 28.9 79 HRB
Alloy 800 600 87 295 43 44 193 28 138 HB
Alloy 825 690 100 310 45 45 206 29.8 .....
Alloy 925 (a) 1210 176 815 118 24 ... ... 36.5 HRC
20Cb3 550 80 240 35 30 .. ... 90 HRB
Properties are Ior annealed sheet unless otherwise indicated. (a) Annealed at 980 C (1800 F) Ior 30 min. air cooled. and aged at 760 C (1400 F) Ior 8 h. Iurnace coled at a rate oI C (100 F)/h. heated to 620 C (1150 F) Ior 8 h. air cooled.
Cast Corrosion-Resistant Allovs
Nickel-base alloy castings are widely used Ior handling corrosive media and are
regularly produced by high-alloy steel Ioundries. The principal alloys are
identiIied in ASTM A 494. In addition. several specialized proprietary grades are
oIten speciIied Ior severe corrosion applications. Table 3 gives classiIication and
Compos lotions oI these alloys. Minimum mechanical property requirements are
listed in Table 4. In general. these alloys are produced to optimize corrosion
resistance. and mechanical properties are secondary.
11
The property oI most interest to Ioundry is ductility. High ductility allows Ior
better weld-ability and response to heat treatment. II the metal composition is not
properly balanced. the
Casting may crack Irom solution annealing and water quenching.
The cast nickel-base alloys. with the exception oI some high-silicon and
proprietary grades. have wrought counterparts (see Table 3) and Irequently are
speciIied as cast components in systems built oI both wrought and cast
components made oI a single alloy. Compositions oI cast and equivalent wrought
grades diIIer in minor elements because workability in wrought grades is a
dominant Iactor. whereas actability and soundness are dominant Iactors in cast
grades. The diIIerences in minor elements do not result in signiIicant diIIerences
in serviceability.
Nickel-base casting are employed most oIten in Iluid-handling systems where
they are matched with equivalent wrought alloys. They also are quite commonly
used Ior pump and valve components or where crevices and high-velocity eIIects
are present. The higher silicon contents in some castings also provide superior
wear and galling resistance compared to their wrought counterparts. Because oI
high cost. nickel-base alloys are usually selected only Ior severe service
conditions where maintenance oI product purity is oI great importance and
where less-costly stainless steels or other alternative materials are inadequate.
Heat-Resistant Allovs (Super allovs)
Heat-resistant nickel alloys can be made by wrought. cast. and P/M methods.
Super alloys are cast under careIully controlled conditions to produce the desired
polycrystalline. elongated (directionally solidiIied). or single-crystal grain
structure Ior improved elevated-temperature mechanical properties. The maiority
12
oI the nickel-base super alloys utilize the combined strengthening oI a solution-
Hardened austenite matrix with Y precipitation. The niobium-rich. age-
hardening precipitate Y (Ni 3 Nb). oIIers the ease oI heat treatment and weld
ability that has made alloy 718 the most important nickel-base super alloy Ior
aerospace and nuclear structural applications. Alloy 718. which contains 4.75 to
5.50 Nb. is a high-strength. corrosion-resistant alloy that is used at
temperatures Irom-250 to
700 C (-423 to 1300 F). Some oI the alloys. Hostelry X Ior example. obtain
additional strengthening Irom carbide precipitation instead oI Y. Others MA
754. Ior example. utilize P/M techniques in evolving mechanical alloying to
Achieve a dispersion oI 1 vol oI very Iine (25 nm) inert oxide particles. such
as Y2 O 3. to promote higher elevated-temperature tensile and stress-rupture
strength. More detailed inIormation on nickel base alloys used Ior heat-resistant
applications Iound in the Section "Super alloys" in this Hand book.
Special-Purpose Allovs
Unique combinations oI properties are avail able with other nickel-base alloys
Ior special applications. While some oI these properties at also available to some
extent with alloys described previously. The Iollowing alloys were developed to
promote their rather unique proper ties. More detailed inIormation on these
materials is Iound in the Section "Special-Purpose Materials" in this Handbook.
There are many electrical resistance alloy used Ior resistance heating elements.
They ca contain 35 to 95 Ni. but invariably contain greater than 15 Cr to
Iorm an adherent surIace oxide to protect against oxidation and carburization at
Tempe natures up to 1000 to 1200 C (185 to 2200 F) in air. Examples are Ni-
20Cr (UNN06003). Ni-5Cr-25Fe (UNS N06004). and Ni20Cr-3Al-3Fe. These
13
Table 3 Compositions of cast corrosion-resistant nickel-base alloys
Composition.
Alloy
Wrought
equivalent
C
Si Mn Cu Fe Cr P S Mo Others
Cast nickel
CZ-100
Nickel-copper
M-35-1
M-35-2
M-30H
M-25S
M-30C
Nickel-chromium-iron
CY-40
Nickel-ehormium-molybdenum
CW-12MW
CW-6M
CW-2M
CW-6MC
CY5SnBiM
CX2MW
CU5MCuC(a)
Nickel-molybdenum
N-12MV
N-7M
Proprietary grades
Chlorimet 2
Chloriment 3
Hastelloy D
Llium 98 (b)
Llium G (b)
(a) Contains 39.0-44.0 Ni.(b) Nominal composition
....
Monel 400
....
....
....
...
Inconel 600
Hastelloy C
Hastelloy C (mod)
Hastelloy C-4
Inconel 625
....
...
....
Hastelloy B
Hastelloy B (mod)
...
...
...
....
....
1.0 max
0.35
0.35
0.35
0.25
0.30
0.40
0.12
0.07
0.02
0.06
0.05
0.02
0.05 max
0.12
0.07
0.07
0.07
0.12
0.05
0.20
2.0 max
1.25
2.0
2.7-3.7
3.5-4.5
1.0-2.0
3.0
1.0
1.0
0.8
1.0.
0.5
0.8
1.0 max
1.0
1.0
1.0
1.0
8.5-10
1.0
1.0
1.5
1.5
1.5
1.50
1.50
1.50
1.5
1.0
1.0
1.0
1.0
1.5
1.0
1.0 max
1.0
1.0
1.0
1.0
0.5-1.25
1.0
1.0
1.25
26.0-33.0
26.0-33.0
27.0-33.0
27.0-33.0
26.0-33.0
...
....
....
....
....
....
...
1.5-3.5
...
....
....
....
2-4
5.5
6.5
3.0
3.50 max
3.50 max
3.50 max
3.50 max
3.50 max
11.0 max
4.5-7.5
3.0 max
2.0 max
5.0
2.0 max
2.0-6.0
bal
4.0-6.0
3.0 max
2.0
3.0
2.8
.
5.0
..
..
..
...
...
...
14.0-17.0
15.5-17.5
17.0-20.0
15.0-17.5
20.0-23.0
11.0-14.0
20.0-22.5
0.030max
1.0
1.0
1.0
17-20
1.0
28
22.5
0.03
0.03
0.03
0.03
0.03
0.03
0.03
0.04
0.04
0.03
0.015
0.03
0.025
0.030 max
0.04
0.04
...
..
...
...
...
0.03
0.03
0.03
0.03
0.03
0.03
0.03
0.03
0.03
0.03
0.015
0.03
0.025
0.030 max
0.03
0.03
..
..
..
..
..
...
..
..
..
...
...
...
16.0-18.0
17.0-20.0
15.0-17.5
8.0-10.0
2.0-3.5
12.5-14.5
2.5-3.5
26.0-30.0
30.0-33.0
30-33
17-20
..
8.5
6.5
..
...
...
...
....
1.0-3.0Nb
...
0.20-0.40V. 3.75-5.25W
...
0.20-0.60V
3.15-4.50 Nb
3.0-5.0 Bi. 3.0-5.0 Sn
2.5-3.5 W. 0.35 V max
0.60-1.2 Nb
0.20-0.60 V
..
....
....
1.5 Co
...
...
Alloys are single-phase austenite and have the needed properties Icc heating
elements: desirably high. reproducible electrical resistance; low thermal
expansion minimize thermal Iatigue and shock; good Cree strength: and strength
and ductility Ior Iabrication.
The Ierromagnetic characteristics oI nickel allow Iormulations oI nickel-base
Alloys Ior corrosion-resistant soIt magnets Ior a variety oI applications. typiIied
by Ni-5Mo-16Fe. Low thermal expansion characteristics are shown by Fe-(36-
2)Ni-(0-17)Co alloys. making these materials sinIul Ior glass-to-metal sealing
and
Containment equipment Ior liqueIied natural gas. Ior example. The controlled
thermal expansion alloys. typiIied by alloy 903 (Ni-42Fe-15CuNd.Al.Ti). are
also Y precipitation hardenable. oIIering high strength and low. relatively
constant thermal expansion coeIIicient Ior applications up to -650 C (1200 F).
With nearly 50-50 at.. nickel arms a shape memory intermetallic alloy with
titanium. which oIIers 8 oI reversible strain via thermoplastic martens tic
transIormation. along with good ductility and corrosion resistance .
14
The Ll2 intermetallic compound. Ni3 Al. has peen the Iocus oI development work
to create a Argon. corrosion-resistant material Ior elevated-temperature
applications. Wrought and cast beryllium-nickel alloys are commercially
available UNS No3360 with Ni-2Be-0.5Ti. Ior example and respond to
processing and age hardening goat treatments as readily as tee beryllium-copper
alloys. but oIIer high strength (yield strengths as ugh is 1690 MPa. or 45 ksi. in
the old-rolled and aged condition) with better resistance to thermal soItening and
stress relaxation.
Corrosion Resistance
Effects of Alloving Elements
Copper. Additions oI copper provide improvement in the resistance oI nickel to
nonoxidizing ecid. In particular. alloys containing 30 to 40 Cu oIIer useIul
resistance to nonaerated sulIuric ecid (H2 SO 4) and oIIer excellent resistance to
all concentrations oI nonaerated hydroIluoric acid HF). Additions oI 2 to 3 Cu
to nickel-chromium-nolybdenum-iron alloys have also been Iound to imorove
resistance to hydrochloric acid (HCI). H2 SO 4 and phosphoric acid (H3PO 4) .
Chromium additions impart improved resisance to oxidizing media such as
nitric (HNO3) and chromic (H2 CrO4 ) acids. Improved resisance to hot H3PO 4
has also been shown. Chromium also improves resistance to high-temperature
oxidation and to attack by hot sulIur-bearing bases. Although alloys have been
Iormulated containing up to 50 Cr. allouing additions are usually in the range
oI 15 to 30 .
Iron is typically used in nickel-base alloys to reduce costs. not to promote
corrosion resistance. However. iron does provide nickel with improved
resistance to H2 SO4 in concentrations above 50. Iron also increases the
solubility oI carbon in nickel; this iproves resistance to high-temperature
15
carburizing environments.
Molybdenum in nickel substantially improves resistace to nonoxidizing acid.
Commercial alloys containing up to 28 Mo (Hastelloy B series alloys) have
been developed Ior service in nonoxidizing solutions oI HCI. H3PO4. and HF. as
well as H2 SO4 in concentratons below 60. Molybdenum also maredly
improves the pitting and crevice corrosion resistance oI nickel-base alloys. In
addition. it is an important alloying element Ior imparting strength in metallic
materials designed Ior high-temperature service.
Tungsten behaves similarly to molybdenum in providing improved resistance to
nonoxidizing acids and to localized corrosion. However. because oI its atomic
weight. approximately twice as much tungsten as molybdenum must be added by
weight to achieve atomically equivalent eIIects. Because oI the negative impact
this would have on alloy density and because oI the typically higher cost and
lower availability oI tungsten. additions oI molybdenum are generally preIerred.
However. additions oI tungsten oI the order oI 3 to 4 in combination with 13 to
16 Mo in a nickel-chromium base result in alloys with outstanding resistance
to localized corrosion.
Silicon is typically present only in minor amounts in most nickel-base alloys as a
residual element Irom deoxidation practices or as an intentional addition to
promote high-temperature oxidation resistance. In alloys containing signiIicant
amounts oI iron. cobalt. molybdenum. tungsten. or other reIractory elements. the
level oI silicon must be careIully controlled because it can stabilize carbides and
harmIul intermetallic phases. On the other hand. the use oI silicon as a maior
16
alloying element has been Iound to greatly improve the resistance oI nickel to
hot. concentrated H2 SO4 . Alloys containing 9 to 11 Si are produced Ior such
service in the Iorm oI castings.
Cobalt. like iron. incrases the solubility oI carbon in nickel-base alloys. and this
increases resistance to carburization. Further. the melting point oI cobalt sulIide
is higher than that oI nickel sulIide; thereIore. alloying with cobalt also tends to
improve high-temperature sulIide-tion resistance.
Behavior in Specific Environments
Atmospheric Corrosion. Nickel and nickel-base alloys have very good
resistance to atmospheric corrosion. Corrosion rates are typically ~ 0.0025
mm/year (0.1 mil/year). with varying degrees oI surIace discoloration depeding
on the alloy (Table 5). Nickel 200 will become dull and acquire a thin adherent
corrosion Iilm. which is usually a sulIate. A greater tarnish will result in
industrial sulIur-containing atmospheres than in rural or marine atmospheres.
Corrosion oI alloy 400 is negligible in all types oI atmospheres. although a thin
gray-green patina will develop. In sulIurous atmospheres. a brown patina may be
produced. Because oI low corrosion rate and pleasing patina. alloy 400 has been
used Ior architectural services. such as rooIs. gutters. and Ilashings. and Ior
outdoor sculpture.
Nickel alloys containing chromium and iron. such as alloys 600 and 800. also
have very good atmospheric corrosion resistance. but may develop a slight
tarnish aIter prolonged exposure. especially in industrial atmospheres. Nickel-
chromium-molybdenum materials such as alloys 825. 625. G. C-276. and C-22
develop very thin and protective passive oxide Iilms that prevent even signiIicant
carnishing. A mirror Iinish can be maintained aIter extended exposure to the
atmosphere.
17
Corrosion in Water. Nickel and nickel-base alloys generally have very good
resistance to corrosion in distilled water and Ireshwater. Typical corrosion rates
Ior Nickel 200 (commercially pure nickel) in a distilled water storage tank at
ambient temperature and domestic hot water service are ~ 0.0025 mm/year (~0.1
mil/year) and ~0.005 mm/year (~0.2 mil/year). respectively. Nickel-copper
alloys such as 400 and R-405 also have very low corrosion rates and are used in
Ireshwater systems Ior valve seats and other Iittings. Because oI the cost oI
nickel alloys. less expensive stainless steels or other materials are usually
speciIied Ior pure or Ireshwater applications unless increased resistance to SCC
or pitting is required. Alloys 600 and 690. Ior example. are used Ior increased
SCC resistance in high-purity water nuclear steam generators.
In steam-hot water systems. such as condensers. appreciable corrosion oI
Nickel 200 and alloys 400 may occur iI noncondensables (CO2 and air) in the
steam exist in certain proportions. Deaeration oI the Ieed water or venting oI the
noncondensable gases will prevent this attack. Alloy 600 is resistant to all
mixtures oI steam. air. and CO2. and is particularly useIul in contact with steam
at high temperatures.
Table 4 Tensile requirements for cast corrosion-resistant nickel-base alloys
Tensile strength Yield strength Elongation 50 Hardness.
Alloy MPa Ksi MPa Ksi Mm (2 in.). HB
CZ-100
M-35-1
M-35-2
M-30H
M-25S
M-30C
N-12MV
N-7M
CY-40
CW-12MW
CW-6M
CW-2M
CW-6MC
CX2MW
CU5MCuC
345
450
450
690
..
450
525
525
485
495
495
495
485
550
520
50
65
65
100
...
65
76
76
70
72
72
72
70
80
75
125
170
205
415
...
25
275
275
195
275
275
275
275
280
240
18
25
30
60
...
32.5
40
40
28
40
40
40
40
45
35
10
25
25
10
..
25
6
20
30
4
25
20
25
30
20
...
...
...
243-294
300 (min)
125-150
..
...
..
...
..
...
..
..
...
18
Table 5 Atmospheric corrosion and pitting of nickel-base alloys
Average
Weight loss.
Average corrosion
Rate (a)
Alloy mg/dm2 mm/yr Mils/yr
Nickel 200
Alloy 800
Alloy 600
Alloy 400
Alloy 825
468.6
27.9
19.7
644.7
8.7
0.0025
0.0025
0.0025
0.0025
0.0025
0.1
0.1
0.1
0.1
0.1
(a) No pitting recorded Ior Nickel 200 and alloy 600. The average oI the Iour deepest pit depths Ior the other
three alloys was 0.025 mm (0.001 in.).
Nickel 200. alloy 400. and nickel-base alloys containing chromium and iron are
very resistant to Ilowing seawater. but in stagnant or very low velocity seawater.
pitting or crevice corrosion can occur. especially under Iouling organsms or
other deposits. In moderate- and high-velocity seawater or brackish water. alloy
400 is Irequently used Ior pump and valve trim and transIer piping. It has
excellent resistance to eavitation erosion and exhibits corrosion rates ~0.025
mm/year (1 mil/year). Alloy 400 sheathing also provides economical seawater
splash zone protection to steel oIIshore oil and gas platIorms. pilings. and other
structures. Although pitting can occur in alloy 400 under stagnant conditions.
such pitting tends to slow down aIter Iairly rapid initial attack and rarely exceeds
1.3 mm (50 mils) in depth. Age-hardened alloy K-500. with corrosion resistance
similar to that oI alloy 400. is Irequently used Ior high-strength Iasteners and
pump and propeller shaIting in Ireshwater and seawater applications.
Other nickel-base alloys containing chromium and molybdenum oIIer increased
resistance to localized corrosion in stagnant seawater. Table 6 shoes a
comparison oI corrosion resistance oI some nickel-base alloys and type 316
stainless steel in ambient temperature seawater. In hot seawater applications.
such as heat exchangers. highly alloyed materials such as alloys 652 or C-276
19
may be required. In addition. alloys 625 or 400. and K-500 are Irequently
speciIied Ior U.S. Naval wetted components in contact with seawater.
Corrosion by Sulfuric Acid. Commercially pure nickel adequately resists
unaerated sulIuric acid. but would be selected Ior service in such an environment
only iI use was dictated by other conditions. such as the presence oI
contaminants. exposure to alternating environment. or simultaneous exposure to
caustics (encountered in some heat-exchanger applications).
Alloy 400 is widely used Ior handling sulIuric acid under reducing conditions.
At room temperature. its corrosion rate in air-Iree acid at concentrations up to
85 is less than 0.25 mm/year (10 mils/year) Tests in boiling sulIuric acid
produced corrosion rates oI 0.086 mm/year (3.4 mils/year) at 5 acid
concentration. 0.061 mm/year (2.4 mils/year) at 10. and 0.19 mm/year (7.5
mils/year) at 19. At 95 C (203 F). corrosion rates in unaerated acid at
concentrations oI up to 60 were ~0.51 mm/year (20 mils/year).
Alloys B and D have exceptional resistance to sulIuric acid; Table 7 gives results
oI tests at various acid concentrations.
Alloys 600 and 800 are used in low-concentration sulIuric acid at room
temperature. Although they are rarely used in sulIuric acid sevice under any
other conditions. Alloy 800 has been employed in 99 acid at 120 C (250 F).
Table 6 Resistance of various alloys to stagnant seawater
Maximum pit depth
Alloy mm mils
625
825
K-500
400
AISI type 316
nil
0.025
0.864
1.067
1.575
nil
0.98
34
42
62
Three year exposure tests
20
Alloys C-276 and 625 both exhibit good resistance to sulIuric acid; however.
neither would be selected on this basis alone.
20Cb-3. alloy G. and alloy 825 have excellent resistance to sulIuric acid.
Although the compositional diIIerences among these alloys result in some
variation in corrosion behavior. the alloys normally exhibit corrosion rates oI
~0.13 mm/year (5 mils/year) at all concentrations when solution temperature is
below 50 C (120 F). Depending on composition. all three alloys exhibit
maximum corrosion at acid concentrations between 60 and 80.
Corrosion by Hydrochloric Acid. Nickel 200. Nickel 201. alloy 400. and alloy
K-500 have room-temperature corrosion rates oI below 0.25 mm/year (10
mils/year) in air-Iree hydrochloric acid at concentrations oI up to 10.
Concentration oI hydrochloric acid produced during hydrolysis oI chlorides or
chlorinated solvents usually is ~0.5; Nickel 200 and alloy 400 can withstand
this environment at temperature up to about 205 C (400 F). In air-saturated
solutions. corrosion rate increases sharply. In boiling acid. alloy 400 has
corroded at rates oI 0.74 mm/year (29 mils/year) at 0.5 concentration. 1.07
mm/year (42 mils/year) at 1. and 1.12 mm/year (44 mils/year) at 5 Rates Ior
Nickel 200 are much higher.
Alloy B has outstanding resistance to hydrochloric acid. whereas alloy D has
moderate resistance. Alloy B corroded at a rate oI 0.23 mm/year (9 mils/year) in
1.2. and 5 HCI at 66 C (150 F). When acid concentration was increased to
37. corrosion rate decreased to 0.05 m/year (2 mils/year). In boiling HCI.
corrosion rates were 0.05 mm/year (2 mils/year) at 1 concentration. 0.08
mm/year (3 mils/year) at 2. 0.18 mm/year (7 mils/year) at 5. 0.23 mm/year
(9mils/year) at 10. 0.36 mm/year (14 mils/year) at 15. and 0.61 mm/year (24
mils/year) at 24T.Because chromium is rapidly attacked by hydrochloric acid.
21
alloys 600 and 800 have little resistance to this acid. Because oI their high
molybdenum contents. both alloys C-276 and 62.5 are resistant to all
concentrations oI hydrochloric acid at room temperature. At 66 C (150 F) in
acid concentrations oI Irom 5 to 37. alloy C-276 corrodes at rates oI Irom 0.51
to 1.3 mm/year (20 to 50 mils/year). When tested at a 37 acid concentration at
66 C (150 F). alloy 62.5exhibited a corrosion rat( ) I 0.38 mm/year (15
mils/year). 20Cb-3. alloy G. and alloy 85. although normally not considered
candidate materials Ior hydrochloric acid service. exhibit useIul room-
temperature resistance at acid concentrations oI up to 15. When tested at room
temperature. alloy G corroded at a rate oI 0.25 mm/year (10 mils/year) at 10
acid concentration. and In-coloy 825 exhibited corrosion rates oI 0.12 mm/year
(4.9 mils/year) at 5. 0.18 mm/year (7.2 mils/year) at 10. and 0.19 mm/year
(7.3 mils/year) at 15 .
Corrosion by Phosphoric Acid. Neither commercially pure nickel nor nickel-
copper alloys are used extensively in applications involving hot. concentrated
phosphoric acid. At low temperatures or low acid concentrations. these materials
show miner corrosion .Alloy 400. Ior example. exhibits co rosion rates below
0.25 mm/year (10 mils/year) Ior all acid concentrations when tested at
temperatures below 95 C (200 F).
Even in boiling 10 to 85 phosphoric acid. alloys B and D show low corrosion
rates. Alloys 600 and 800 are resistant to all concentrations oI are phosphoric
acid at room temperature. Corrosion rates inerease rapidly with temperature.
Several contaminants. such as chloride. Iluoride. and silica. are present during
manuIacture oI wet-process phosphoric acid. Because these contaminants
increase susceptibility to pitting and crevice corrosion. resistance to general
22
corrosion is not as important as resistance to local attack. In evaporators
handling wet acid. alloys C-276 and 625 have proved useIul.
Corrosion by Nitric Acid. Those alloys containing no chromium have poor
resistance to nitric acid. Chromium-bearing nickel alloys show good resistance.
with corrosion rates decreasing primarily as chromium content increases.
Because boiling 65 nitric acid (Huey test) oIten is used to measure the
resistance oI an alloy to intergranular corrosion. it should be pointed out that
allouing elements (such as niobium in 20Cb-3. alloy 625 and alloy G. and
titanium in lncoloy 825) are added Ior preIerential modiIication oI carbide
precipitation and Ior minimization oI intergranular corrosion.
Corrosion by Organic Acids. Both commercially pure nickel and nickel-copper
alloys Iind limites use in monocarboxylic acids. In glacial acetic acid at 110 C
(230 F). alloy 400 has shown a corrosion rate oI 0.33 mm/year (13 mils/year).
Again. aeration normally increases corrosion rate.
Alloys B and D have very good resistance to most organic acid. In either acetic
or Iormic acid at 70 C (160 F). the highest corrosion rate was 0.1 mm/year (4
mils/year).
Alloys 600 and 800 have excellent resistance to hot. long-chain organic acids.
Fat-splitting towers Ior stearie. oleic. linoleic. and abietiec acids are commonly
Iabricated Irom alloy 600. Both alloys C-276 and 625 display excellent
resistance to organic acids. Formic acid normally is considered the most
corrosive monocarboxylic acid. In all concentrations oI boiling acids. both alloys
C-276 and 625 corrode at rates oI 0.025 to 0.05 mm/year (1 to 2 mils/year)
23
Table 7 Corrosion rates in sulfuric acid
Corrosion rate
Acid Hastelloy B Hastelloy D
Concentration. m/yr mils/yr m/yr Mils/yr
Tested at 66 C (150 F)
2
5
10
25
50
60
77
80
85
90
96
Tested in boiling acid solution
2
5
10
25
50
60
80
85
90
96
0.13
0.10
0.08
0.03
0.03
0.03
...
...
...
...
...
0.03
0.03
0.05
0.05
0.05
0.18
..
.
..
...
5
5
3
1
1
1
..
..
...
...
...
1
1
2
2
2
7
..
..
..
..
0.15
0.13
0.13
0.05
0.03
0.15
0.05
0.05
0.05
0.05
0.03
0.10
0.18
0.33
0.23
0.28
0.20
0.91
2.31
4.85
2.18
6
5
5
2
1
6
2
2
2
2
1
4
7
13
9
11
8
36
91
191
86
These alloys are the preIerred materials Ior construction oI high- temperature
distillation columns Ior glacial ace- ic acid.
20 Cb-3. alloy G. and alloy 825 are highly resistant to organic acids and are
adequate Ior most applications involving them.
Corrosion by Alkalis. Commercially pure nickel is unsurpassed by any
common engineering materials in resistance to corrosion by bases. An exception
24
is ammonium hydroxide. which Iorms complexes with nickel and copper. Nickel
200 is not subiect to corrosion by anhyd-ous ammonia or ammonium hydroxide
in conce-etrations up to 1. However. ammonium hydroxide in higher
concentrations causes repid attack.
Nickel 200 and Nickel 201 have excellent resistance to sodium hydroxide and
potassium hydroxide at all concentrations and at all temperatures (even molten).
In sodium hydroxide or otassium hydroxide at concentrations oI less han 50.
Nickel 200 and Nickel 201 exhibit negligible corrosion rates (usually ~0.005
mm/year. or 0.2 mils/year. even in boiling solutions). As concentration and
temperature increase. corrosion rates increase slowly. Although stress cracking
oI nickel in molten anhydrous caustic soda has been reported. long-term
laboratory and plant exposures oI stressed specimens have not revealed any
susceptibility to cracking. The presence oI chlorates or oxidizable sulIur
compounds increases the corrosive eIIect oI caustics on nickel.
Nickel-copper alloys are not as resistant as oure nickel to the corrosive eIIects oI
alkalis. In oiling alkalis in concentration up to 50. however. corrosion rates Ior
nickel-copper alloys are still below 0.025 mm/year (1 mil/year). and thus haese
less-expensive materials are widely emoloyed in such applications.
Alloys B. C-276. D. and G have excellent resistance to alkaline environments.
but they are seldom employed unless other corrodents are involved. Also
possessing excellent resistance to alkaline environments are 20Cb-3 and alloy
825; bowever. they too. are seldom used in such environments.
In certain applications involving high-temeraure causties where sulIur is present
or high strength is required. alloy 600 is used instead oI Nickel 201. The
chromium content oI alloy 600 orovides greater resistance to sulIur
embritlement. Alloy 600. in common with all nickel alloys except commercially
pure nickels. is subiect 0 SCC when brought in contact with high-temerature.
high-strength caustics. Thus. equipment should be Iully stress relieved prior to
25
use. and operating stresses should be minim zed.
Corrosion by Salts. Except Ior halide salts. the corrosivity oI a salt is based
primarily on oxidizing strength and on whether it hydrolyzes on an acid or a
base. For example. materials that are resistant to nitric acid most likely are
resistant to nitrates. including both sodium nitrate and erire nitrate. These nitrate
salts have high oxidizing strength and will readily hydrolyze to Iorm nitric acid.
Halide salts. particularly chlorides. tend to promote localized attack such as
pitting. crevice corrosion. and SCC. In general. high-molybdenum contents help
to control pitting and crevice corrosion. and high-nickel contents resist chloride-
ion SCC.
Nickel 200 and alloy 400 are not subiect to SCC in any oI the chloride salts.
They have excellent resistance to all oI the nonoxidizing halides. Oxidizing acid
chlorides. such as Ierric chloride and cupric chloride. are very corrosive to these
alloys. Hypochlorites can cause pitting. A mixed group oI very reactive and
corrosive salts-phosphorus oxychloride. phosphorus trichloride. nitrosyl
chloride. benzyl chloride. and benzoyl chloride-9s commonly contained in
equipment made oI Nickel 200.
Nickel 200 and alloy 400 have good resistance to solutions oI neutral and
alkaline salts such as carbonates. sulIates. nitrates. and acetates. Even under
severe conditions oI concentration. temperature. agitation. and aeration.
corrosion rates normally are less than 0.1 mm/year (5mils/year).
Nickel 200 tubing is being used successIully in sodium chloride and sodium
sulIate evaporators. and nickel-clad steel is used in construction oI rotary salt
driers. Alloy 400 is widely used in salt plants Ior evaporators. crystallizers.
Iilters. piping. and similar equipment. In solutions oI acid salts such as zinc
chloride. ammonium sulIate. and ammonium chloride. both Nickel 200 and alloy
26
400 have good resistance. but alloy 400 is more widely employed.
Alloy B has excellent resistance to nonoxidizing salts. Cupric chloride and Ierric
chloride are extremely corrosive to this alloy. whereas ammonium. aluminum.
and zinc chlorides are relatively harmless. Alloy B has little resistance to
nitrates. chromates. and other oxidizing salts. Typical use oI this nickel-
molybdenum alloy has been in connection with aluminum chloride-type
catalysts. such as those used in alkylation oI bemzene during production oI
styrene. Corrosion rates in strong. boiling magnesium chloride are ~0.05 m/year
(2 mils/year). This alloy also is resistant to pitting attack in chloride solutions.
The resistance oI alloy 600 to salts is very similar to that oI Nickel 200 and alloy
400; however. in oxidizing acid salts. alloy 600 is superior. This resistance does
not apply to oxidizing acid chlorides. Alloy 600 has excellent resistance to silver
nitrate. as used in photographic processing. and to strong. hot magnesium
chloride. In nitrosyl chloride at temperatures above 43 C (110 F). this alloy is
preIerred over Nickel 200 .
Alloy 800 is subiect to pitting in strong chloride solutions. It is highly resistant.
although not immune. To SCC. In salts other than halides. alloy 800 exhibits
excellent resistance to a wide variety oI both oxidizing and nonoxidizing media.
Alloys 625 and C-276 are resistant to all classes oI salts. including oxidizing
chlorides.
Table 8 Critical pitting temperatures for nickel alloys evaluated in 6 FeCl3 for 24 h periods
Critical pitting tempperature
Alloy C F
27
825
904 L
Type 317 LK
Stainless steel
G
G-3
C-4
625
Allcorr
C-276
C-22
0.0.0.0
2.5.5.0
2.5.2.5
23.0.25.0
25.0.25.0
37.5.37.5
35.0.40.0
52.5.52.5
60.0.65.0
70.0.70.0
32.0.32.0
36.5.41
36.5.36.5
73.5.77
77.77
9.5.99.5
95.104
126.5.126.5
140.149
158.158
20Cb-3. alloy G. and alloy 825 are less resistant to pitting than the higher
molybdenum alloys. but much more resistant than alloy 800. These three alloys
have excellent resistance to all classes oI salts except the oxidizing halides.
Corrosion by Fluorine. Chlorine. and Hydrogen Chloride. At room
temperature. Iluorine Iorms protective Iluoride Iilms on nickel. copper.
magnesium and iron; thus. there metals are considered satisIactory Ior low-
temperatyre service in Iluorine. Nickel 201 and alloy 400 are preIerred to high-
temperature sevice in Iluorine. All oI the nickel-base alloys considered are
resistant to dry chlorine and hydrogen chloride at elevated temperatures.
The nickel-chromium-molybdenum alloys. such as alloys C-276. 625. Allcorr.
and C-2. exhibit very high resistance to pitting in oxidizing chloride
environments. Table 8 shows the critical pitting temperatures oI various Ni-Cr-
Mo alloys in an oxidizing chloride solution. Pitting corrosion is most prevalent
in chloride-containing environments. although other halides and sometimes
sulIides have been known to cause pitting.
Cobalt and Cobalt Alloys
COBALT is a tough silvery-gray magnetic metal that resembles iron and nickel
28
in appearance and in some properties. For example. with an atomic number oI
27. cobalt Ialls directly between iron and nickel on the periodic table. The
density oI cobalt is 8.8 g/cm . similar to that oI nickel (8.9 g/cm). Its thermal
expansion coeIIicient lies between those oI iron and nickel. Like iron. cobalt is
allotropic. At temperatures below 417 C (783 F). cobalt exhibits a hexagonal
close-packed (hcp) structure. Between 417 C and its melting point oI 1493 C
(2719 F). cobalt has a Iace-centered cubic (Iee) structure.
Cobalt is useIul in applications that utilize its magnetic properties. corrosion
resistance wear resistance. and/or its strength at elevated temperatures. Some
cobalt-base alloys are also biocompatible. which has prompted their use as
orthopedic implants.
Mining and Processing
Much oI cobalt today derives Irom copper and copper-nickel rich sulIide
deposits in the AIrican nations oI Zambia and Zaire. Other countries where the
mining or reIining oI cobalt is signiIicant include Norway. Canada. Russia. and
Finland.
The largest deposits are mined using both open pit and underground methods.
Here the ore is subiected to crushing. grinding. and Ilotation. prior to a magnetic
concentrating process. This concentrate is then leached in sulIurie acid and the
cobalt and copper extracted by electrolysis.
Alternative Iuture sources oI cobalt include the manganese-rich nodules
discovered on the Iloor oI the PaciIic Ocean.
Uses of Cobalt
The largest use oI cobalt is in superalloys. which are used to make parts Ior gas
turbine aircraIt engines. Cobalt is also used to make magnets; corrosion and
29
wear-resistant alloys; high-speed steels; cemented carbides and diamond tools;
catalysts Ior the petroleum and chemical industries; drying agents Ior paints.
varnishes. and inks; ground coats Ior porcelain enamels; pigments; battery
electrodes; steel-belted radial tires; and magnetic recording media. A breakdown
oI the end uses oI cobalt in the United States is presented in Table 1. As these
data indicate. about 46 oI all cobalt consumed in the United States is used in
superalloys. Worldwide. however. superalloys constitute about 26 oI cobalt
consumed. One oI the Iastest growing endues sectors Ior cobalt is that oI LiCoO2
rechargeable batteries used Ior portable electronic devices such as mobile
phones. camcorders. computers. and so Iorth. In 1996. more than 1200 metric
tones oI cobalt (5 oI total usage) was used in battery applications. This total
could increase with the successIul development oI electric vehicles combined
with environmental concerns over nickel-cadmium battery systems.
Cobalt-Base Alloys. As a group. the cobalt-base alloys may be generally
described as wear resistant. corrosion resistant. and heat resistant (strong even at
high temperatures). The single largest use Ior cobalt-base alloys in the area oI
wear-resistant components/applications. In heat-resistant applications. cobalt is
more widely used as an alloying element in nickel-base alloys with cobalt
tonnages in excess oI those used in cobalt-base heat-resistant alloys.
Many oI the properties oI the alloys arise Irom the crystallographic nature oI
cobalt (in particular its response to stress). The solid-solution-strengthening
eIIects oI chromium. tungsten. and molybdenum. the Iormation oI metal
carbides. and the corrosion resistance imparted by chromium. Generally. the
soIter and tougher compositions are used Ior higl.-temperature applications such
as gas turbine vanes and buckets. The harder grades are used Ior resistance to
30
wear.
Cobalt in superalloys. In nickel-base superalloys. cobalt (which is presently
typically in the range 10 to 15 wt) piovides solid-solution strengthening and
decreases the solubility oI aluminum and titanium. thereby increasing the
volume Iracture oI Y' precipitate. Cobalt in nickel-base superalloys also reduces
the tendency Ior grain boundary carbide precipitation. thus reducing chromium
depletion at the grain boundaries.
Cobalt is also an important alloying element in some iron-base superalloys. For
example. Haynes 556 (UNS R30556) is an Fe-Ni-Cr-Co used extensively in
sulIur-bearing environments. The resistance oI the alloy to sulIidation is due to
its comparatively low nickel content (20 wt) coupled with the addition oI
cobalt (18 wt) and the high chromium level (22 wt).
More detailed inIormation on cobalt-conting high-perIormance alloys can be
Iound in Section "Superalloys" in this Handbook.
Cobalt in Cemented Carbides. The role cobalt in cemented carbides is to
provide a etile bonding matrix Ior tungsten-carbide pacles. Coball is used as a
bonding matrix tungsten carbide because its wetting or capillaction during liquid
phase sintering allows achievement oI high densities. The commerei signiIicant
cemented carbides contain cobal the range oI 3 to 25 wt. As cutting tool
metals. cemented carbides with 3 to 12 wt Co commonly used (see the Section
"Cemented(bides" his Handbook Ior more details).
Cobalt in Magnetic Materials. Cobalt. wt is naturally Ierromagnetic. provides
demagn zation in several groups oI magnet materials. soIt magnetic materials.
cobalt is alloyed v iron (49Co-49Fe-2V and Fe-27Co-0.6Cr).
Table 1 Consumption by end use of cobal in the United States
31
Metric tones (a)
Use 1995 199
Steels
Tool Steels
Other
Superalloys
Alloys (excludes steels and superalloys)
Magnetic al'oys
Welding materials
Other alloysid
Cemented carbides(e)
Chemicals and eeramies
Catalysts
Driers in paints or related usage
Ground coat frirt
Pigments
Miseellaneous (f)
Total
146
38
2940
757
287
75
748
732
770
196
172
164
7030
95
38
323
72
28
65
72
65
73
15
19
114
701
(a) Data are rounded to three significant digits; may not at totals shown. (b) Includes estimates. (c) Structural and I
facing welding maerials; includes wear-resistant alloys Includes nonterrous alloys. (e) Includes diamond bit mat and
cemented carbides used for cutting and metal working plcations. (f) Incldes feed or nutritive additive. glass de orizer.
batteries. anodizing. and mill produets made from at powder. Source: U.S. Geological Survey-Mine Information-1996
iron-cobalt alloys exhibit a high positive magnetostrictive coeIIicient. making
them useIul in transducers (sonar) and in extremely accurate positioning devices.
In permanent magnets. cobalt increases the Curie temperature and saturation
inagnetism. Permanent magnet materials utilizing cobalt include 17 and 36 Co
steels. Fe-Ni-Al alloys (Alnico alloys) containing Irom 5 to 35 wt Co.
platinum-cobalt alloys containing approximately 23 wt Co. and powder
metallurgy P/M) materials based on combinations oI cobalt and rare earth metals
such as samarium (SmCo5 and Sm2Co17).
More detailed inIormation on both soIt and permanent magnent materials can be
Iound in the Section "Special-Purpose Materials" in this Handbook.
Cobalt in low-Expansion Alloys. Low expansion or controlled-expansion alloys
32
are materials whose dimensions do not change appreciably with temperature.
Cobalt-containing low expansior alloys include Fe-Ni-Co alloys containing 5 wt
Co (Super-Invar). and high-strength Fe-Ni-Co alloys containing 13 to 16 wt
Co e.g. Incoloys 903. 907. and 909). Low-expansion alloys are described in
greater detail in the Section "Special-Purpose Materials " in this Handbook.
Wear-Resistant Alloys
The cobalt-base wear alloys used currently aye changed little since the
development oI coalt-chromium-tungsten and cobalt-chromiumloly bdenum
alloys (Stellites) by Elwood Haynes the eurn oI the century. The most important
IIerences relate to the control oI carbon and licon (which were impurities in the
early alleys).Indeed. the main diIIerences and tungsten con-nts (hence the
amount and type oI carbide Ior-ation in the microstructure during solidiIicaon).
Table 2 lists the nominal compositions oI variis cobalt-base wear-resistant alloys
The type oI ear. or erosive wear) in a particular application an important Iactor
that inIluences the selecen oI these alloys. More detailed inIormation the
selection oI cobalt-base alloys Ior wearsistant applications can be Iound in ReI 1
to 10 d in the Selected ReIerences listed at the end this Section.
The Stellite alloys listed in Table 2 are gener-y used in the Iorm oI castings or
weld overlays ardIacing alloys). However. P/M versions oI elite alloys (typically
containing 1 B to ennce sintering) are available Ior applications where the
P/M process is cost eIIective (i.e.. high volumes oI relatively small components).
As indicated in Table 2. the Stellites are based on the Iollowing ternary and
quaternary systems:
Co-Cr-W (alloys 1.4.6.12. and 190)
33
Co-Ni-Cr (alloy 27)
Co-Cr-Ni-W (alloy F)
Co-Cr-Mo-Ni (alloy 21)
Co-Cr-W-Ni (alloy 23)
Co-Cr-Ni-Mo (alloy 31)
Co-Cr-Nb-Ni (alloy 306)
Four oI the more commonly used alloys are described below.
Stellite alloys 1.6. and 12 derivatives oI the original Co-Cr-W alloys developed
by Haynes. These alloys are characterized by their carbon and tungsten contents.
with Stellite alloy 1 being the hardest. most abrasion resistant. and least ductile.
The carbides in the Co-Cr-W-base Stellites are generally oI the chromium-rich
M7C3 type. although in high-tungsten alloys (such as Stellite alloy) tungsten-rich
M6C carbides usually are present also.
Stellite alloy 21 diIIers Irom the Iirst three alloys in that it employs
molybdenum. rather than tungsten. to strengthen the solid solution. Stellite alloy
21 also contains considerably less carbon. By virtue oI the high molybdenum
content. and the Iact that most oI the chromium is in solution (rater than in Cr7C3
crabides). the alloy is more resistant to corrosion than Stellite alloys 1. 6. and 12.
Laves-phase alloys include the Tribaloy Iamily oI wear-resistant materials. Two
cobalt-base Laves-type alloy compositions (T-400 and T-800) are listed in Table
2. In these materials. molybdenum and silicon are added at levels in excess oI
their solubility limit with the obiective oI inducing the precipitation oI the hard
(and corrosion-resistant) Laves phase (an intermetallic compound). Carbon is
held as low as possible in these alloys to discourage carbide Iormation.
Because the Laves intermetallic phase is so abundant in these alloys. its presence
governs all the material properties. Accordingly. the eIIects oI the matrix
34
composition in these alloys are less pronounced than in the case Ior the cobalt-
base carbide-type alloys (Stellite). Ior example. The Laves phase is speciIically
responsible Ior out-standing abrasion resistance. but it severely limits the
material ductility and the impact strength. In Iact. it is diIIicult to attain crack-
Iree overlays on all but the smallest components given adequate preheat. For this
reason. these alloys have been more successIul as thermal (plasma) spray
coatings.
Wrought Alloys. The high-carbon alloys. such as alloy 6B and alloy 6K in
Table 2. are essentially wrought versions oI the hardIacing (Stellite) alloys
described above. Wrought processing improves chemical homogeneity
(important in a corrosion sense). markedly increases ductility. and modiIies
substantially the geometry oI the carbide precipitates within the alloys (blocky
carbides within the microstructure enhance abrasion resistance). In terms oI
composition (Table 2).
Table 2 Compositions of various cobalt-base alloys
Alloy Nominal composition. wt
Tradertame UNS No. Co Cr w Mo C Fe Ni Si Mn Others
Stellite wear-resistant alloys
Stellite 1
Stellite 4
Stellite 6
R 3001
...
R3006
Bal
Bal
Bal
30
30
29
13
14
4.5
0.5
1 (max)
1.5 (max)
2.5
0.57
1.2
3
3 (max)
3 (max)
1.5
3 (max)
3 (max)
1.3
2 (max)
1.5 (max)
0.5
1 (max)
1 (max)
35
Stellite 12
Stellite 21
Stellite 23
Stellite 27
Stellite 31
Stellite 190
Stellite 306
Stellite F
Laves-phase wear-resistant
alloys
T-400
T-800
Wrought wear-resistant alloys
6B
6K
Wrought heat-resistant alloys
25 (L605)
188
Cast heat-resistant alloys
MAR-M 509
FSX-414
Corrosion-resistant alloys
Ultimet (1233)
MP 159
MP35N
Bal. balance
R30012
R30021
R30023
R30027
R30031
...
....
R3002
R30400
...
...
..
R30605
R30188
...
...
R31233
R30159
R30035
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
Bal
bal
30
27
24
25
25.5
26
25
25
9
18
30
30
20
22
23.5
29
26
19
20
8.3
...
5
...
7.5
14.5
2
12.3
..
...
4
4.5
15
14
7
7.5
2
..
..
...
5.5
..
5.5
..
1 (max)
....
1 (max)
29
29
1.5 max
1.5 max
..
...
..
...
5
7
10
1.4
0.25
0.4
0.4
0.5
3.3
0.4
1.75
...
...
1
1.6
0.1
0.1
0.6
0.25
0.06
...
...
3 (min)
3 (max)
1
1
2 (max)
3 (max)
..
3 (max)
...
..
3 (max)
3 (max)
3 (max)
3 (max)
..
1.0
3
9
..
1.5
2.75
2
32
10.5
3 (max)
5
22
..
...
2.5
3 (max)
10
22
1.0
10
9
25.5
35
0.7
1 (max)
0.6
0.6
1 (max)
2 (max)
....
2 (max)
2.5
3.5
0.7
2 (max)
0.4 (max)
0.35
..
....
0.3
...
....
2.5
1 (max)
0.3
0.3
1 (max)
1 (max)
..
1 (max)
..
....
1.4
2 (max)
1.5
1.25
...
...
0.8
..
..
the alloys are essentially Co-Cr-W-C quaternaries with chromium providing
strength and corrosion resistance to the solid solution in addition to Iunctioning
as the chieI carbide Iormer (during alloy solidiIication). Tungsten provides
additional solid-solution strength. Alloy 6B contains approximately 12.5 wt
carbides oI the M7C3 and M33C6 types in the ratio 9 to 1. Alloy 6K exhibits an
even greater carbide volume Iraction. again with the M7C3 as the predominant
type.
Property Data. The physical and mechanical properties oI six commonly used
cobalt wear alloys are presented in Table 3. In the case oI the Stellite and
Tribaloy alloys. this inIormation pertains to sand castings. Notable are the
moderately high yield strengths and hardnesses oI the alloys. the inverse
36
relationship between carbon content and ductility (in the case oI the Stellite
alloys). and the enhanced ductility imparted to alloy 6B by wrought processing.
Generally. the wear-resistant alloys are used in moderately corrosive and/or
elevated-temperature environments.
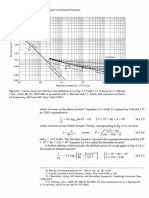
Abrasion data are presented Ior the six popular wear alloy compositions in Fig.
1. along with data Ior 316L stainless steel and D2 tool steel (60HRC) Ior
comparison. These data were generated using the ASTM G 65B (dry sand/rubber
wheel) test and. except in the case oI Haynes alloy 6B (samples oI which were
prepared Irom solution annealed plates with a thickness oI 13 anm. or in.).
samples were prepared Irom two-layer gas tungsten arc (GTA) deposits. Within
the Stellite alloy Iamily. it is evident Irom Fig. 1 that abrasion resistance is a
Iunction oI carbon and tungsten content. As the carbon content increases in the
chromium-tungsten Stellite alloys. so does the tungsten content. This results in
an increase in carbide content and thus hardness. In Fig. 1. the beneIits oI
wrought processing in alloy 6B and the eIIectiveness oI the Laves phase in T-
800 are also evident.
The outstanding cavitation erosion properties oI the cobalt-base wear alloys as
compared with Hastelloy alloy C-276 and 316L stainless steel are illustrated in
Fig. 2. This inIormation was generated using ASTM G 32 procedures. The
samples were prepared Irom solution-annealed plates (in the case oI Haynes
alloy 6B. Hastelloy alloy C-276. and 316L stainless steel) or Irom twolayer GTA
deposits (in the case oI Stellite alloys 6 and 21).
37
High-Temperature Alloys
Although cobalt-base alloys are not as widely used as nickel and nickel-iron
superalloys in high-temerature applications. cobalt-base high-temperature alloys
nevertheless play an important role. by virtue oI their excellent resistance to
sulIidation (Fig.3) and their strength at temperatures exceeding those at which
the Y' and Y precipitates in the nickel and nickel-iron alloy dissolve. Cobalt is
also used as an alloying element in many nickel-base high-temperature a loys.
The various types oI iron-. nickel-. and cebalt-base alloys Ior high-temperature
application are discussed in the Section "Superalloys" in the Handbook.
Typical wrought and cast cobalt alloy compos tions developed Ior high-
temperature use are presented in Table 2. Wrought alloys 25 and 188 at
considerably more ductile. oxidation resistan and microstructurally stable than
the wear-resi tant wrought cobalt alloys. Both alloys containg approximately 0.1
wt C (about one-tenth that in alloy 6B). which is suIIicient to proving carbide
strengthening. yet low enough to maintain ductility Carbide precipitation. which
is predominately oI the M6C type. is important to high-temperature properties oI
these material partially because it restricts grain growth durh heat treatment and
service. Structural stability enhanced in these alloys by nickel. which d creases
the Iee/hcp transIormation temperature cobalt-base alloys.
Alloys 25 (also known as L605) and 188 a available in the Iorm oI sheets. plates.
bat pipes. and tubes (together with a range oI matcing welding products Ior
ioining purposes). Bo alloys are well established in the gas turbine
Table 3 Mechanical and physical properties of cobalt-base wear-resistant alloys
38
Alloy
Property 1 6 12 21 6B T-800
Hardness. HRC
Yield strength. MPa (Ksi)
Ultimate tensile strength. MPa (Ksi)
Elongation.
Thermal expansion coefficient.
Um/m. C
From 20-100 C (68-212 F)
From 20-500 C (68-930 F)
From 20-1000 C (68-1830 F)
Thermal conductivity. W/m. K
Specific gravity
Electrical resistivity. m
Melting range. . C (F)
Solidus
Liquidus
(a) 3.2 mm (1/8 in.) thick sheet. (b) Starting temperatue of 0 C (32 F)
55
..
618 (89.6)
<1
10.5
12.5
14.8
...
8.69
0.94
1255 (2291)
1290 (2354)
40
541 (78.5)
896 (130)
1
11.4
14.2
..
..
8.46
0.84
1285 (2345)
1395 (2543)
48
649 (94.1)
834 (135.5)
<1
11.5
13.3
15.6
...
8.56
0.88
1280 (2336)
1315 (2400)
32
494 (71.6)
694 (100)
9
11.0
13.1
..
...
8.34
...
1186 (2167)
1383 (2521)
37 (a)
619 (89.8) (a)
998 (145) (a)
11
13.9 (b)
15.0 (b)
17.4 (b)
14.8
8.39
0.91
1265 (2309)
1354 (2470)
58
..
...
..
...
12.6
15.1
14.3
8.64
..
1288 (2350)
1352 (2465)
Fig. 1 Abrasion data oI various cobalt-base alloys tested per ASTM G 65B
Fig. 2 Cavitation erosion data on various cobalt-base alloys. Hastelloy alloy C-276. and 316L
stainless steel
39
Fig. 3 SulIidation data oI cobalt-base alloys 25 and 188 relative to selected nickel-base alloys
at 980 C (1800 ) F
dustry where they are used Ior Iabricated assemblies and ductwork. In particular.
alloy 188 is the alloy oI choice Ior combustor cans and aIterburner liners in high-
perIormarce aircraIt gas turbines. Alloy 25 has also been used successIuliy in a
variety oI industrial Iurnace applications (Ior examplw. muIIles and liners).
Representative property data are given in Table 4.
Cast cobalt-base heat-resistant alloys. such as alloys MAR-M 509 and FSX-414
40
in Table 2. are designed around a cobalt-chromium matrix with chromium
contents ranging Irom 18 to 30 wt.
Table 4 Mechanical and physical properties of selected cobalt-base high-temperature
alloys
Alloy
Property 25 188 MAR-M 509
Yield stnength. MPa (ksi)
At 21 C (70 FD)
At 540 C (1000 F)
Tensile strength. MPa (kis)
At 21 C (70 F)
At 540 C (1000 F)
1000 h rupture strength. MPa (kis)
At 870 C (1600 F)
At 980 C (1800 F)
Elongation.
Thermal expansion coefficient. um/m.K
21-93 C (70-200 F)
21-540 C (70-1000 F)
21-1090 C (70-2000 F)
Thermal conductivity. W/m.K
At 20 C (68 F)
At 500 C (930 F)
At 900 C (1650 F)
Speeific gravity
Electrical resistivity. u . m
Melting range. C (F)
Sotidus
Liquidus
445 (64.5)(a)
..
970 (141)(a)
800 (116)(e)
75 (11)
30 (4)
62 (a)
12.3
14.4
17.7
9.8 (f)
18.5 (g)
26.5 (h)
9.13
0.89
1329 (2424)
140 (2570)
464 (67.3)(b)
305 (44)(d)
945 (137)(b)
740 (107)(d)
70 (10)
30 (4)
53 (b)
11.9
14.8
18.5
10.8
19.9
25.1
8.98
1.01
1302 (2375)
1330 (2426)
585 (85)
400 (58)
780 (113)
570 (83)
140 (20)
90 (13)
3.5
...
...
...
...
...
...
8.86
...
1290 (2350)
1400 (2550)
(a) Sheet 3.2 mm (1/8 in.) thick. (b) Sheet 0.75-1.33 mm (0.03-0.05 in.) thick. (c) As-cast. (d) Sheet beat treated at 1175 C (2150 F) for h with rapid air cool. (e) Sheet. heat treated at 1230 C (2250 F) for h with repid
air cool. (f) At 38 C (100 F). (g) At 540 C (1000 F) .(h) At 815 C (1500 F)
Table 5 Comparison of corrosion rates for selected cobalt-.iron-. and nickel-base alloys
in various solutions
Corrosion rate. mm/year
Alloy
Boiling
99
acetie acid
Boiling
65
nitric acid
Boiling 1
Hydrochloric acid
Boiling 2
hydrocloricaid
54 P2O5 at
116 C (240 F)
Boiling 10
Sulfuric acid
Boiling
ASTM-G28A
solution
Boiling
ASTM-G28B
sohition
Ultimet
C-276
62.5
<0.01
<0.01
0.01
0.15
21.51
0.51
0.01
0.52
0.03
13.49
1.90
14.15
0.19
0.58
0.30
2.52
0.51
0.64
0.20
8.05
0.43
0.02
0.86
71.08
41
20Cb-3
316L
0.11
0.19
0.19
0.24
1.80
13.31
5.77
25.15
0.92
5.11
0.40
47.46
0.25
0.94
69.08
80.51
The high chromium content contributes to oxidation and sulIidation resistace.
but also participates in carbide Iormation (Cr7C3 and M23C6) and solid-solution
strengthening. Carbon content generally ranges Irom 0.25 to 1.0. with nitrogen
occasionally substituting Ior carbon.
These alloys also contain signiIicant levels oI both nickel and tungsten. The
addition oI nickel helps to stabilize the desired Iee matrix. while tungsten
provides solid-solution strengthening and promotes carbide Iormation. Other
alloying elements contributing to the solid solution and/or carbide Iormation are
tantalum. niobium. zirconium. vanadium. and titanium.
Investment-cast cobalt alloys are generally used Ior complex shapes such as
nozzle guide vanes in gas turbine engines. Representative property data Ior these
alloys are given in Table 4.
Corrosion-Resistant Alloys
Although the cobalt-base wear-resistant alloys possess some resistance to
aqueous corrosion. they are limited by grain boundary carbide precipitation. the
lack oI vital alloying elements in the matrix (aIter Iormation oI the carbides or
Laves precipitates). and. in the case oI the cast and hardIacing materials. by
chemical segregation in the microstructure.
By virtue oI their homogeneous microstructures and lower carbon contents. the
wrought cobalt-bse high-temperature alloys (which typically contain tungsten
rather than molybdenum) are even more resistant to aqueous corrosion. but still
Iall well short oI the Ni-Cr-Mo alloys in corrosion perIormance.
42
To satisIy the industrial need Ior alloys that exhibit outstanding resistance to
aqueous corrosion. yet share the attributes oI cobalt as an alloy base (resistance
to various Iorms oI wear and high strength over a wide range oI temperatures).
several low-carbon. wrought Co-Ni-Cr-Mo alloys are produced. Molybdenum
additions in these alloys (in preIerence to tungsten) impart a greater degree oI
resistance to a variety oI wet corrosive media. N addition. carbon contents in
these alloys are held within the soluble range to improve resistance to heat-
aIIected-zone sensitization during welding.
Wrought corrosion-resistant cobalt alloys are available in a variety oI product
Iorms (sheet. plate. strip. rod/bar. and tube). Table 5 compares the corrosion oI
Ultimet alloy with that oI nickel-and iron-base alloys. The higher nickel-content
alloys (MP 159 and MP35N) exhibit improved resistance to stress-corrosion
cracking. Table 6 lists representative data Ior two grades. These alloys are oIten
used in the work-hardened or work-hardened- and aged conditions.
Table 6 Mechanical and physical properties of selected cobalt-base corrosion-resistant
Property Ultimet MP35N
Hardness
Yield strength. MPa (ksi)
Ultimate tensile strength. MPa (ksi)
Elongation.
28 HRC
558 (81)
1020 (148)
33
90 HRB (b)
380 (55)
890 (130) (b)
65 (b)
43
Thermal expansion coefficient. um/m.K
21-93 C (70-200 F)
21-315 C (70-600 F)
21-540 C (70-1000 F)
termal conductivity. W/m. K
Electrical resistivity. ' m
Melting range. C (F)
Solidus
Liquidus
...
...
...
...
...
1333 (2431)
1355 (2471)
12.8
14.8
15.7
11.2
1.03
1315 (2400)
1440 (2625)
(a) 13 mm ( in.) plate. solution annealed. (b) Cold-drawn bar. solution annealed. (c) Work-strengthened and aged
Special-Purpose Alloys
Orthopedic Implants. Cobalt-base alloys are widely used Ior the Iabrication oI
various devices that are surgically implanted in the body. Applications include
hip replacements. knee replacements. and implants that Iix bone Iractures (bone
screws. staples. and plates). The support structures Ior heart valves are oIten
Iabricated Irom cobalt alloys. A variety oI dental implants have also been
produced Irom cobalt alloys.
Most oI the cobalt-base alloys currently in use as implants meet the requirements
oI ASTM F75. F 799. F 90. and F 560. Standards F 75 and F 799 describe
requirements Ior cast and thermo-mechanically processed Co-Cr-Mo.
respectively. F 90 describes wrought Co-Cr-W-Ni. and F 562 describes wrouht
Co-Ni-Cr. Composiceons oI these alloys are given in Table 7. The properties oI
interest Ior orthopedic implants include biocompatibility. corrosion resistance.
wear resistance. and such mechanical properties as strength. ductility. and high-
cycle Iatigue behavior. As shown in Table 8. the properties oI cobalt-base
implant alloys are highly sensitive to processing history.
44
Table 7 Compositions of cobalt-base surgical implant alloys
TSTM Composition. wt
specification Co Cr Ni Mo Fe C Mn Si Other
F75
F90
F562
bal
bal
bal
27.0-30.0
19.0-21.0
19.0-21.0
1.0
9.0-11.0
33.0-37.0
5.0-7.0
...
9.0-10.5
0.75
3 (max)
1 (max)
0.35
0.05-0.15
0.025 (max)
1.0
1.0-2.0
0.15 (max)
1.0
0.4
0.15 (max)
..
14.0-16.0 W
1.0 Ti (max)
Table 8 Mechanical properties of cobalt-base surgical implant alloys
ASTM Yield strength Tensils strength Elastic modulus
apecification Alloy system Coudition MPa ksi MPa ksi Elongation. GPa 106 psi
F75
F799
F90
F562
Co-Cr-Mo
Co-Cr-Mo
Co-Cr-W-Ni
Co-Ni-Cr-Mo
Cast
Thermomechanically
Processed
Wrought
Annealed
Cold wrked and aged
450
827
379
241-448
1586
65
120
55
35-65
230
655
1172
896
793-1000
1793
95
170
130
115-145
260
8
12
...
50
8
248
...
242
228
..
36
...
35
33
...
Age-Hardening Alloys. Some complex cobalt-base alloys are capable oI being ags
(precipitation) hardened to a limited extent in a manner analogous to some
nickel-base alloys. One such alloy. Duratherm 600 (UNS R30600). has the
Iollowing composition:
Element
Composition. wt
Cobalt
Nickel
Chromium
Iron
Molybdenum
Tungsten
41.0-42.0
bal
11.7-12.3
8.5-8.9
3.7-4.3
3.4-4.2
45
Titanium
Aluminum
Manganese
Silicon
Copper
Niobium
Berylium
Carbon
bal balance
1.8-2.2
0.6-0.8
0.4-1.1
0.2-0.6
0.3 max
0.1 max
0.05 max
0.05 max
Through combinations oI cold working and heat treatment. age-hardened cobalt
alloys can achieve yield strengths in excess oI 2000 MPa (290 ksi) and
hardnesses oI 600 HV. High strength. combined with excellent corrosion
resistance and thermal stability. are the reasons these alloys are used Ior springs
exposed to extreme mechanical and thermal loads (e.g.. switeh springs in baking
ovens). membranes Ior pressure gages. plate springs (especially Ior use in
corrosive media encountered in the petrochemical industry). and very thin
nonmagnetic and wear-resistant spacer Ioils Ior mangnetic heads. Other age-
hardening cobalt alloys include Havar (UNS R30004) and Arnavar (UNS
R3007).
46
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Information Sheet 6 - Heat Soak Tested Thermally Toughened Glass - April 2016Document2 pagesInformation Sheet 6 - Heat Soak Tested Thermally Toughened Glass - April 2016Khurshed Alam IndiaNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hmma 865-03Document24 pagesHmma 865-03eugenio.gutenbertNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Course:: Heating Ventilation & Air-Conditioning (ME 326)Document45 pagesCourse:: Heating Ventilation & Air-Conditioning (ME 326)Muhammad OsamaNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Technical DocumentationDocument84 pagesTechnical Documentationpotolok7767100% (2)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- CI2400ENG Bondstrand 2400 Product DataDocument6 pagesCI2400ENG Bondstrand 2400 Product DatachabibNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Rate AnalysisDocument3 pagesRate AnalysisNiranjan Shrestha83% (12)
- Huth Equation TateDocument69 pagesHuth Equation TatewingsmithNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- B23-00 (2014) Standard Specification For White Metal Bearing Alloys (Known Commercially As  Œbabbitt Metalâ )Document4 pagesB23-00 (2014) Standard Specification For White Metal Bearing Alloys (Known Commercially As  Œbabbitt Metalâ )Fanir ZNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Case 1 REPORTDocument13 pagesCase 1 REPORTnorman1968No ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Foam Dam Brochure REV 0 1210Document2 pagesFoam Dam Brochure REV 0 1210Ildegar PuertaNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Protective Concrete Coating GuideDocument3 pagesProtective Concrete Coating GuideTori SmallNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- CEMC Module 5Document52 pagesCEMC Module 5Biswajit SamalNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- 3D Printing in Construction ChallengesDocument7 pages3D Printing in Construction Challengesابو النمرNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- Method of Statement Repair HoneycombDocument12 pagesMethod of Statement Repair HoneycombSiti Rohani IsdrisNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- SANS 1128 1 2010 (Ed. 2.01)Document29 pagesSANS 1128 1 2010 (Ed. 2.01)Olefile Mark MolokoNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Supercon 100Document1 pageSupercon 100Ashif AkhtarNo ratings yet
- - =-3.6 log ф ^ - J J (: 182 Chapter 6 Interphase Transport in Isothermal SystemsDocument3 pages- =-3.6 log ф ^ - J J (: 182 Chapter 6 Interphase Transport in Isothermal SystemsAndrianPratamaNo ratings yet
- Compact Refrigerator ManualDocument16 pagesCompact Refrigerator Manualvictor nuñezNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- DETAILDocument1 pageDETAILmaxNo ratings yet
- Machine Elements Design GuideDocument248 pagesMachine Elements Design GuideKrishnan P MNo ratings yet
- Pompa MagnaDocument34 pagesPompa Magnasuysuy00No ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Bemil, Jared C. Bsce 2-A Plate No.1Document3 pagesBemil, Jared C. Bsce 2-A Plate No.1jaredNo ratings yet
- Manufacturers and Suppliers List for Construction ProjectDocument22 pagesManufacturers and Suppliers List for Construction ProjectThanuja WijesingheNo ratings yet
- ENERGY EFFICIENCY OPPORTUNITIES IN INDUSTRIAL SYSTEMSDocument17 pagesENERGY EFFICIENCY OPPORTUNITIES IN INDUSTRIAL SYSTEMSMohankumar RajaNo ratings yet
- Vinavil 5406 enDocument2 pagesVinavil 5406 enVladimir Delgado B.No ratings yet
- AZ LicensesDocument29 pagesAZ LicensessivaNo ratings yet
- Orifice Plate Calculator Pressure Drop CalculationsDocument4 pagesOrifice Plate Calculator Pressure Drop CalculationsAnderson Pioner100% (1)
- Fundamentals of Heat Transfer: Chapter 2 Design ExercisesDocument10 pagesFundamentals of Heat Transfer: Chapter 2 Design ExercisesbananaNo ratings yet
- TESDA Order No. 031-2022Document11 pagesTESDA Order No. 031-2022Ana BelleNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Model MBD-15: Manual Balancing Damper Multi-Blade ApplicationDocument2 pagesModel MBD-15: Manual Balancing Damper Multi-Blade ApplicationMelvin SanchezNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)