Professional Documents
Culture Documents
Replication Mammalian 2009
Uploaded by
Leda TorresOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Replication Mammalian 2009
Uploaded by
Leda TorresCopyright:
Available Formats
DNA Replication: Mammalian
Brandy M Snider, Indiana University School of Medicine, Indianapolis, Indiana, USA Elizabeth A Phipps, Indiana University School of Medicine, Indianapolis, Indiana, USA Shanna J Smith, Indiana University School of Medicine, Indianapolis, Indiana, USA Brittney-Shea Herbert, Indiana University School of Medicine, Indianapolis, Indiana, USA Robert J Hickey, Indiana University School of Medicine, Indianapolis, Indiana, USA Linda H Malkas, Indiana University School of Medicine, Indianapolis, Indiana, USA
Based in part on the previous version of this Encyclopedia of Life Sciences (ELS) article, DNA Replication: Mammalian by Robert J Hickey, Derek J Hoelz and Linda H Malkas.
Advanced article
Article Contents
. Introduction . Proteins that Drive the Mammalian DNA Replication Fork . Proteins Involved in Leading Strand Synthesis . Proteins Involved in Lagging Strand Synthesis . Complex Actions are Required for Mammalian Cell DNA Replication Initiation . Mammalian DNA Replication at the Telomere . DNA Replication and Mammalian Cell Nuclear Architecture . The Nuclear Matrix . The Role of the Nuclear Matrix in DNA Synthesis . DNA Replication Factories . Summary and Future Directions
Online posting date: 15th December 2009
It is well known that deoxyribonucleic acid (DNA) replication occurs at the replication fork, whereby the parent strand of DNA unwinds and two daughter strands are formed, which are subsequently synthesized in a leading and lagging strand manner. Furthermore, many proteins and enzymes are involved in initiation of DNA replication, and the synthesis of DNA, as well as the structural component of the nuclear matrix, which allows for attachment of replication proteins. It is also known that replication of mammalian DNA is a very complicated and highly regulated process, requiring strict regulation, along with many different proteins and enzymes. Although there is a wealth of information regarding basic DNA synthesis, there is still much more information to learn regarding the machinery involved in initiation of DNA replication, DNA synthesis and, ultimately, DNA replication.
Introduction
The process of deoxyribonucleic acid (DNA) replication in mammalian cells is highly complex and has several unique features that distinguish it from simpler prokaryotic systems. First, chromosomal DNA replication is
compartmentalized within the mammalian cell nucleus and is partitioned from the cytoplasm, which is the site of synthesis of proteins and other metabolites that function in DNA synthesis, as well as the site for the mediation of extracellular stimuli that may trigger the initiation of DNA replication. Second, the mammalian chromosome is a complex nucleoprotein structure composed of both DNA and protein. These chromosomal-associated proteins must be duplicated along with the DNA to maintain proper chromosomal organization that in turn inuences gene expression. Third, the mammalian chromosome contains multiple replication origins per DNA molecule that promote the initiation of DNA synthesis in a precise and temporally regulated manner. The precise ring of these replication origins leads to a spatially regulated chromosomal DNA synthesis occurring in dened replication units or replicons. Therefore, in that mammalian cells contain multiple chromosomes as well as many replication origins on a single chromosome, the act of chromosomal DNA replication must be a highly coordinated process. In this article the current knowledge regarding the proteins mediating mammalian DNA replication is described. There is also a discussion of the role the mammalian cell nuclear architecture plays in DNA replication.
Proteins that Drive the Mammalian DNA Replication Fork
Since the DNA molecule codes the information necessary for life, it is very important that replication be accurate and highly regulated. In 1953, Watson and Crick set forth their hypothesis regarding DNA replication using a template model (Watson and Crick, 1953). In the subsequent years, a variety of studies were performed that supported their template model premise. One important experiment by Meselson and Stahl (1958) described how the parent DNA strand unwinds into two chains which are each used as a template to make two daughter DNA molecules (reviewed
1
ELS subject area: Biochemistry How to cite: Snider, Brandy M; Phipps, Elizabeth A; Smith, Shanna J; Herbert, Brittney-Shea; Hickey, Robert J; and Malkas, Linda H (December 2009) DNA Replication: Mammalian. In: Encyclopedia of Life Sciences (ELS). John Wiley & Sons, Ltd: Chichester. DOI: 10.1002/9780470015902.a0001041.pub2
ENCYCLOPEDIA OF LIFE SCIENCES & 2009, John Wiley & Sons, Ltd. www.els.net
DNA Replication: Mammalian
in Alberts, 2003). Another key study involved the discovery of the enzyme DNA polymerase, which uses a parent DNA strand as a template from which to synthesize a daughter strand (reviewed in Alberts, 2003). Later DNA replicationbased experiments revealled that replication occurs at a Yshaped junction on DNA, called the replication fork (reviewed in Alberts, 2003). On initiation of DNA replication, several proteins and enzymes are recruited to the replication fork which promotes synthesis of the leading and lagging DNA strands (Trujillo and Osley, 2008). The proteins identied thus far as involved in mammalian cell DNA replication include: the MCM helicase complex, DNA polymerase a (pol a) primase, proliferating cell nuclear antigen (PCNA), replication factor C (RFC), DNA polymerase d, ap endonuclease 1 (FEN1),
Table 1 Proteins involved in the mammalian DNA replication fork Protein MCM complex Topoisomerase I and II RPA DNA polymerase a primase PCNA RFC Pol d FEN1 RNAse H DNA ligase I Function Helicase to unwind the duplex DNA Reduces torsional strain during unwinding ssDNA-binding protein prevents refolding Synthesizes RNA primer DNA processivity factor (DNA clamp) Clamp loader Replicative polymerase Removes RNA primer ap Removes RNA primer Ligates Okazaki fragments
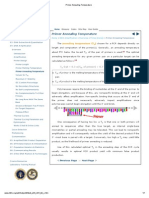
replication protein A (RPA), DNA ligase I, topoisomerase I and II, and RNAse H (ribonucleic acidase H) (see Table 1; Alberts, 2003; Hickey et al., 2003; Trujillo and Osley, 2008). A model depicting their action at the mammalian DNA replication fork is described in Figure 1.
Proteins Involved in Leading Strand Synthesis
Leading and lagging strand synthesis is initiated and managed by the formation of the replisome and its associated proteins at the replication fork (Alberts, 2003). However, before strand synthesis can occur, the minichromosome maintenance (MCM) assembly factor must be converted into a replicative helicase by the Cdc45 (cell division cycle)/GINS (Go, Ichi, Nii, San) complex (Forsburg, 2008). Once the MCM complex is converted into a helicase, it begins to unwind the DNA with the help of DNA topoisomerases to reduce torsional strain, and thereby generating small areas of ssDNA (single-stranded DNA) (Alberts, 2003; Forsburg, 2008). RPA, the ssDNAbinding protein, is loaded onto the ssDNA after unwinding, and moves along the replication fork, to prevent the DNA from reforming into the double helix (Forsburg, 2008). Once unwound, the DNA is made accessible to other proteins involved in strand synthesis, including pol a-primase, PCNA, RFC and pol d (Forsburg, 2008). The pol aprimase synthesizes an RNA primer that uses complementary base pairing to associate with the ssDNA. RFC is the clamp-loading protein that allows PCNA to encircle DNA. In an ATP (adenosine triphosphate)-expending process, three molecules of PCNA are loaded onto the DNA, forming a clamp that encircles the DNA (Trujillo
Leading strand
PCNA Pol Topo I&II MCM Pol primase RPA
RN Ase H
DN liga A se I
RFC
PCNA
RFC
FEN
Lagging strand
Figure 1 Model of mammalian cell DNA replication fork. ENCYCLOPEDIA OF LIFE SCIENCES & 2009, John Wiley & Sons, Ltd. www.els.net
DNA Replication: Mammalian
and Osley, 2008). PCNA/RPA function to tether the replicative polymerase pol d to the DNA, thereby acting as a polymerase switch displacing the primase with polymerase, to further process the new strand of DNA (Forsburg, 2008; Trujillo and Osley, 2008). With the pol d tethered to the DNA, DNA synthesis can occur in long segments, since the polymerase does not have to dissociate from the strand (Walther and Wold, 2001). Replication of the leading strand is continuous, occurs in the 5!3 direction, and is always growing towards the replication fork. See also: Eukaryotic Replication Fork
Proteins Involved in Lagging Strand Synthesis
Owing to the antiparallel nature of DNA replication, the lagging strand is synthesized in a much dierent manner than the leading strand. The lagging strand is discontinuous and is always synthesized away from the replication fork. It is synthesized in a series of short fragments called Okazaki fragments (Rossi et al., 2006). Pol a-primase synthesizes an RNA primer at the 5 end of each Okazaki fragment. After the initial synthesis by the primer, polymerase switching occurs, whereby the pol a is removed and pol d binds (Rossi et al., 2006). The pol d then adds nucleotides to the 5 end which further extends the Okazaki fragments to the 5 end of the previously synthesized Okazaki fragment (Alberts, 2003). When the two fragments converge, a single-stranded ap is formed when the RNA primer is displaced (Rossi et al., 2006; Aguilera and Gomez-Gonzales, 2008). This ap is then cleaved by FEN1 and RNAse H resulting in a nick, which is subsequently, closed by DNA ligase I (Rossi et al., 2006; Aguilera and Gomez-Gonzales, 2008). Continuous double-stranded DNA results.
Complex Actions are Required for Mammalian Cell DNA Replication Initiation
DNA replication is a committed step of cell division and as such stringent checkpoint controls exist to ensure it is initiated once per cell cycle and only after any necessary repairs are made to the template DNA. Though replication initiation demarcates the onset of S phase, the manner and timing by which this complex event occurs is largely determined in G1. In mammalian cells, it is during G1 that ORC (origin recognition complex) binds multiple replication origins and recruits Cdc6, Cdt1 (chromatin licensing and DNA replication factor 1) and MCM proteins to form pre-replication complexes. The decision to activate specic pre-replication complexes in an organized fashion and initiate S phase is made only after rigorous assessment of protein levels, DNA delity and epigenetic environment.
Under the right conditions, an irreversible choice to replicate culminates in the temporally orchestrated ring of multiple origins, and, at the conclusion of S phase, the faithful duplication of the chromosome. The eort to unravel the complexities of the pre-programming that occurs in G1 and leads to replication initiation has fuelled the discovery of new interacting partners for previously identied initiation factors. Work of this nature focused on p21 and led to the discovery of Ciz1 (p21Cip1 interacting zinc-nger protein). It has been demonstrated that Ciz1 RNAi (RNA interference) knock-down mammalian cells assembled components of the pre-replication complex but did not initiate replication eciently (Coverly et al., 2005). The role of Cdt1 in replication initiation is likely also more complex than initially thought. It is well established that Cdc6 and Cdt1 independently bind ORC, setting the stage for the subsequent recruitment of MCM 27 proteins. A new study postulates MCM 9 forms a stable association with Cdt1 and is required at prereplication complexes for MCM 27 binding to occur (Lutzmann and Mechali, 2008). Other recent bodies of work provide evidence that proteins involved in transcription also help determine the patterned ring of S phase origins. Along this line, MYC, an oncogene most well known for its role in binding enhancers and recruiting HATs (histone acetyltransferases) to facilitate opening of chromatin structure, may bind DNA and signal factors involved in replication initiation leading to selective ring of origins. This notion was based on the nding that higher levels of MYC protein corresponded to a greater number of actively ring origins (Cole and Cowling, 2008). Previous reports have implicated the AP-1 complex (containing proteins of the c-fos, c-jun, activating transcription factor (ATF) and Jun dimerization protein 2 (JDP) families) as having a role in driving G1 to S transition. Consistent with this, Cadoret et al. (2008) recently used ndings from ENCODE, a cooperative study aimed at determining functional elements of the human genome, and Chip-on-Chip analysis and discovered binding sites for either c-fos, c-jun, or both in over half of the 283 previously unknown origins they identied. As technological advances enable genome-wide analysis, characterization of less well-studied stretches of DNA lends to our ability to make generalizations about the genetic and epigenetic components of origins and how these factors regulate initiation. Cadoret et al. (2008) identied histone-modication sites at origins consistent with open chromatin structure. This nding was not surprising as it has long been reported that open chromatin structure was favoured at origins and promoter regions to facilitate the loading of molecular machinery. However, these modication sites were only seen in half of the origins studied, suggesting open chromatin structure does not determine replication initiation. Another study (Gimenes et al., 2008) reported open chromatin structure at mammalian origins in the form of an alternate, bent DNA conformation. The authors hypothesize that, like histone
3
ENCYCLOPEDIA OF LIFE SCIENCES & 2009, John Wiley & Sons, Ltd. www.els.net
DNA Replication: Mammalian
modications, this feature facilitates ORC recognition and binding. Considerable eort has been devoted to interpreting how genomic and epigenetic environment of origins translates into a timed pattern of ring. It has long been known that chromatin state plays an integral role in origin selection and temporal ring with the general assumption being that euchromatin correlates with earlier ring in S phase than heterochromatin. Previous studies have also shown that early replicating regions of the genome have a high CpG density. Cadoret et al. (2008) also discovered a higher density of origins in CpG-rich regions as compared to CpG-poor regions. As a result of this nding, the researchers controlled for CpG content and asked whether origin density alone was predictive of temporal ring but found no correlation.
Mammalian DNA Replication at the Telomere
The evolutionary decision to package DNA into linear chromosomes presents a unique set of challenges; namely, replication results in incomplete synthesis of the lagging strand. This incomplete synthesis dilemma is commonly referred to as the end-replication problem, rst described by James Watson and Alexi Olovnikov in the early 1970s (Sfeir et al., 2005; Takeda and Dutta, 2005). Although leading strand synthesis is continuous, lagging strand synthesis is discontinuous and based on a series of Okazaki fragments that are ligated to each other (Bianchi and Shore, 2008). Since DNA polymerase can only initiate synthesis in the 5!3 direction with a preformed primer, it cannot fully synthesize the 3-terminal end of the lagging strand of linear DNA. As a result, each round of DNA replication is met with loss of lagging strand DNA at the chromosome ends due to the gap left behind after removal of the last RNA primer. The replication machinery cannot ll this gap because there is no DNA ahead of the removed primer to initiate synthesis. The outcome is an end-replication problem in which terminal DNA is lost with each round of division (Sfeir et al., 2005). To combat this, noncoding DNA evolved at the chromosome ends to act as a buer and protect the rest of the encoding genome from degradation. The end of the chromosome was described as a telomere (its name derived from the Greek nouns telo, meaning end, and mere, meaning part). Below a critical threshold, the continued shortening of the telomeres induce an irreversible cell growth arrest (senescence), cell death or genomic instability (Chakhparonian and Wellinger, 2003). Telomeres also help maintain the integrity of the genome by preventing end-to-end fusions of chromosomes and degradation. Mammalian telomeres consist of many kilobases of 5TTAGGG/3-AATCCC tandem DNA repeats that are complexed as chromatin with nucleosomes and several regulatory, protective proteins (e.g. TRF1, TRF2,
4
protection of telomeres 1 (POT1), TRF-1 interacting protein 2 (TIN2), repressor/activator protein 1 (Rap1), tripeptidyl peptidase 1 (TPP1), collectively referred to as the shelterin or telosome) (Bianchi and Shore, 2008; Gilson and Geli, 2007; and references within). By forming repressive subtelomeric chromatin, human telomeres also display a telomere position eect in which gene expression is inhibited. Telomeric repeats vary highly in number among mammalian species and within dierent cell types of the same organism (515 kb in humans, up to 100 kb in some mice). Leading strand synthesis copies the C-rich strand continuously and generally results in blunt-ended DNA. However, lagging strand synthesis of the G-rich strand results in a 3 overhang that varies in length from approximately 35400 nucleotides in mammalian cells. The variance in overhang length could be due to the position of the RNA primer before it was removed after DNA replication or end-processing events. The 3 overhang has been shown by electron microscopy to tuck back onto itself, displace the double-stranded telomere (known as the D-loop), resulting in a T loop. T loops are known to be present on both ends of the chromosome, suggesting that processing of the blunt end from the leading strand synthesis also occurs to result in overhangs and formation of a T loop. This T loop, in addition to the binding of shelterin proteins, oers further protection from the fusion of chromosome ends. Telomeres can be extended by the RNAprotein complex known as telomerase (reviewed in Olovnikov, 1973). To carry out DNA replication at telomeres, the telomerase complex is equipped with a catalytic subunit TERT (the RNA-dependent DNA polymerase, or reverse transcriptase, of the telomerase complex) and an RNA template component, TERC (or TR). Expression of TERT was once thought to be limited to cancerous, developing, stem and germline cells. However, TERT is now thought to exist at very low levels during S phase in dierentiated normal cells, presumably because of the growth advantage and protection against DNA damage and cell death that telomerase may provide. Levels in normal cells, however, are not high enough to lead to telomere lengthening or continual maintenance. In cells with moderate or high levels of telomerase, telomerase is preferentially recruited to the shortest telomeres at individual chromosomes. In other words, telomerase activity is tightly regulated to allow only enough DNA synthesis to partially oset the base pair loss incurred by the end-replication problem, thus preserving genomic stability. Recent evidence even suggests that telomerase may be linked to the DNA synthesis machinery used elsewhere in the genome, such as at sites of DNA damage or breaks. However, in approximately 10% of cells with continuous lifespan yet without TERT or TERC gene expression, telomeres can be lengthened by a recombination-based approach, known as the alternative lengthening of telomeres (ALT) pathway. The generation of the overhangs and determination of the last base on each strand has been extensively studied in model organisms, suggesting that two processing steps are
ENCYCLOPEDIA OF LIFE SCIENCES & 2009, John Wiley & Sons, Ltd. www.els.net
DNA Replication: Mammalian
involved for the G- and C-rich strand, respectively. However, studies of the last base in mammalian cells have been more dicult due to varying length of overhangs and telomeres. Recently developed assays to measure the overhang length control in human cells (see Bianchi and Shore, 2008) have aided in the understanding of mammalian telomeric DNA replication. As stated earlier, lagging strand synthesis results in 3 G-rich overhangs, whereas leading strand synthesis results in blunt ends or endprocessing to form 5 C-rich overhangs leading to further end-processing. Recent analysis showed that 80% of the chromosomes end in ATC-5 on the C-strand (Bianchi and Shore, 2008). This last base has been observed at both ends, suggesting that overhang processing is common for both strands of replication. The last base of the G-rich strand is less precise, but at least 70% of the strands have been observed to show a preference for TAG-3 (at 40%), TTA3 and GTT-3. Taken together, these three sequences are indeed predicted to occur if the C-strand template for leading strand replication is 3-CCAATC-5, with the possibility for the replication complex to dissociate one or two nucleotides before the end (for TTA-3 and GTT-3). In the presence of telomerase, 50% of chromosomes ended in the TAG-3 sequence, which matches the last base of the RNA template region of telomerase, as well as indicating that no further end-processing occurred at the G-rich end. The end-processing of telomeres to form 3-overhangs and T loops at both ends suggests the presence of resecting nucleases. However, a base-specic nuclease for the telomere has not yet been identied. Current nucleases of interest include those involved with general lagging strand DNA synthesis. For example, the 5!3 exonuclease and 5 ap endonuclease FEN1, in conjunction with PCNA and DNA polymerase-d/e, are involved with removal of RNA primers, Okazaki fragment processing and maturation (Bianchi and Shore, 2008). In addition, the ReqQ helicases Werner syndrome protein (WRN) and Bloom syndrome protein (BLM) are required for ecient lagging-strand replication of the G-rich telomeric DNA by dissociating the unusual DNA structures during replication, to prevent dramatic loss of telomeric DNA. These nucleases have been linked to telomere stability (e.g. WRN and FEN1) as well as being regulated by shelterin proteins, such as TRF2 (Bianchi and Shore, 2008 and references within). DNA replication at telomeres is undoubtedly subject to unique regulation and timing. The replication timing of mammalian telomeres diers between dierent species (Gilson and Geli, 2007). In human cells, telomere replication has been observed to occur throughout S phase; however, subtelomeric region replication occurs during late S phase. Recently, detailed observations of replication forks at the telomere suggest that completion of telomere replication may be deferred to late S phase and coordinated with other cell cycle events. Fork progression through the chromosome ends in mammalian cells can be hindered by heterochromatin and complex DNA structures present at the telomere. The single-stranded, G-rich overhangs present at the telomeres can form GG base paired tetrastrand
structures (G-quartets or G-quadruplexes) that have been shown to hinder progression of the replication fork, as well as inhibit telomerase in vitro. The shelterin telomeric protein complex and T loop structure at the telomere can also prevent fork progression and accessibility or recruitment of telomerase to the telomere. The search for replication origins at mammalian telomeres is ongoing; however, several reports show that primase can bind to telomeres and their replication machinery (i.e. telomerase) in yeast and ciliate model systems (see Gilson and Geli, 2007). The binding of primase to telomeres may aid in coordinating G-strand synthesis by telomerase, whereas the DNA replication machinery synthesizes the C-rich lagging strand. Finally, regulation of telomere replication can occur through epigenetic modications at telomere that may limit telomerase function in certain cell types. Telomerase expression itself can be regulated at the transcriptional, epigenetic, translational and posttranslational level, as well as by other binding proteins within the complex. Therefore, the cell allows itself to have many levels of tight regulation for the replication of telomeres to maintain genome stability and cell survival.
DNA Replication and Mammalian Cell Nuclear Architecture
Nuclear architecture plays an essential role in the process of synthesizing mammalian DNA, which has been evidenced in numerous studies. Nuclear architecture is a term used to describe structures such as the nuclear matrix and the organization of DNA replication factories. A description of these structures will be detailed in the sections that follow.
The Nuclear Matrix
Ultrastructural studies within the nuclei of mammalian cells have revealed an extensive network of euchromatin, heterochromatin and nonchromatinous laments. These networks are further organized into functional domains, providing sites for DNA replication and transcription, RNA processing and ribosome biogenesis (Philimonenko et al., 2004). In 1974, Berezney and Coey rst described this subcellular structure as the nuclear matrix. Since then, much has been discovered about the nuclear matrix and its replication function (Berezney and Coey, 1974). The nuclear matrix was rst identied over 50 years ago, when extraction studies of nuclei using high-salt solutions resulted in an insoluble residual fraction: this portion resisted extraction even after treatment with buers at high ionic strength. Recently, the framework of the nuclear matrix has been visualized using both electron microscopy (Anachkova et al., 2005; He et al., 1990; Wan et al., 1999) and atomic force microscopy (AFM) (Wan et al., 1999). Electron microscopy revealed a diuse network of
5
ENCYCLOPEDIA OF LIFE SCIENCES & 2009, John Wiley & Sons, Ltd. www.els.net
DNA Replication: Mammalian
irregular and intricately structured bres. These bres consist of 913 nm thick core laments mostly covered with ribonucleoprotein. The matrix is considered to join together with RNA in a type of three-dimensional internal brogranular network through the nucleus and intersecting the peripheral lamina. Using AFM on treated HeLa cells, the brous network of the nuclear matrix was revealed (Wan et al., 1999). The nuclear matrix is most likely situated within the interchromatin (IC) space occupying the nucleus of living cells. This placement, however, is hard to conceptualize because of the vast dierence in structure: the IC space consists of a sinusoidal system bounded by chromatin contours, whereas the nuclear matrix consists of a crisscrossing network of straight laments. To account for this discrepancy, it has been proposed that the nucleus contains a number of compartments of short lament arrays, which results in a number of local nuclear matrices strategically placed (Hirano et al., 2008). These local regions would harbour a distinct set of proteins, resulting in several specialized nuclear matrices. Over 400 dierent proteins have been identied as having an association with the nuclear matrix. These proteins include enzymes, structural proteins, transcription, RNA processing and replication factors (Martelli et al., 2002). Many of these proteins are categorized as associated with the internal nuclear matrix, whereas others have matrix association dependent on the cell type or cell cycle (Martelli et al., 2002). Although the function of each protein is known, the how and why of their nuclear matrix association remain undened.
The Role of the Nuclear Matrix in DNA Synthesis
Experimental evidence implicates the involvement of the nuclear matrix in numerous aspects of DNA replication, including: initiation, chromosomal DNA replication and regulation. In fact, the nuclear matrix may be involved in unwinding of the DNA double helix, allowing for attachment of the machinery for DNA synthesis, and causing easy separation of the daughter DNA molecules from the parental template during replication. At the present, however, there is no conclusive evidence for a specic matrix-binding region on the daughter DNA molecule, or specic attachment sites for DNA on the nuclear matrix. The replication process is thought to begin when a sequence-specic DNA-binding protein (initiator) binds to a specic genome region (replicator). This initiator would subsequently enlist other proteins that unwind the DNA and assemble the replisome (Cvetic and Walter, 2005). The ORC is thought to exist in a six-subunit protein form. Over 20 mammalian sites of origin have been identied, but, at this point, no specic sequences have been determined. Recent studies have revealed a cell cycle-dependent association of the ORC with the nuclear matrix; this complex
6
favourably attaches to the nuclear matrix late in the G1 phase (Djeliova et al., 2001a, b; Ohta et al., 2003). This could create functionally active pre-replication foci during attachment to the nuclear matrix, resulting in replication initiation at the nuclear matrix (Radichev et al., 2005). In separate experiments, chromatin fractions have been isolated from Chinese hamster ovary (CHO) cells synchronized late in the G1 phase. This fraction contained approximately 30% chromosomal DNA attached to the nuclear matrix, which acted as a substrate for the initiation of DNA replication with human HeLa cells synchronized in S phase (Dijkwel et al., 1986). Biochemical and immunouorescent microscopy showed that the chromatin fraction attached to the nuclear matrix initiated DNA replication. In fact, it was possible to initiate replication on isolated matrix preparations in the absence of chromatin (Anachkova et al., 2005). DNA in the earliest stages of replication are attached to the nuclear matrix, and it has been proposed that only DNA attached to the nuclear matrix is able to correctly initiate DNA synthesis using a specic replication origin sequence. Additional experiments utilized autoradiographic analysis of nuclear matrix halo structures to track the positioning of a DNA replication origin relative to the nuclear matrix (Nakamura et al., 1984). Using synchronized BHK (baby hamster kidney) cells, DNA labelled before S phase remained matrix-associated and migrated into the DNA halo later. This further implies that the replication origins remain matrix-bound after the initiation of DNA synthesis. Finally, experimental data suggests that the nuclear matrix is a key component in the organization of DNA as well as a factor in regulating the initiation of DNA synthesis by using a specic DNA sequence (Anachkova et al., 2005).
DNA Replication Factories
As described earlier, the nuclear matrix is a highly organized structure that provides binding sites for both DNA and RNA during synthesis. In fact, evidence from numerous experiments leads to the possibility that DNA replication occurs in replication factories that are associated within the nuclear matrix. A normal replication focus is a cluster that contains several nearby replicons that re simultaneously in S phase and contain an average of 1 Mb of DNA (Martelli et al., 2002). The stable localization of the replication foci in the nucleus is a strong indicator of their attachment to the nuclear matrix. These sites for replication have been visualized using rat broblasts in S phase incubated with bromodeoxyuridine. By using uorescently labelled antibodies against the analogue, these focal sites were revealed (Nakayasu and Berezney, 1989). Fluorescent microscopy has been used to further visualize the nuclear matrix and replication factories. Studies on human cells using a monoclonal antibody recognizing
ENCYCLOPEDIA OF LIFE SCIENCES & 2009, John Wiley & Sons, Ltd. www.els.net
DNA Replication: Mammalian
DNA polymerase a with indirect immunouorescence microscopy show a granular pattern of uorescence in the cell nuclei (Nakayasu and Berezney, 1989). This type of pattern is a characteristic of the nuclear matrix. Furthermore, orescent microscopy was used to map DNA replication sites in the mammalian interphase cell nucleus with the addition of biotinylated dUTP (deoxyuridine triphosphate; Jackson and Cook, 1986a). These distinct areas were distributed throughout the nuclear interior and along the periphery. From this, it was suggested that each granular visualized corresponded to a replication cluster assembly, where a set of arranged replicons are coordinately synthesizing DNA. As another way to more carefully examine the replication sites, an extremely sensitive electron microscopy localization procedure was followed. In this case, biotin16-dUTP was incorporated in vivo, followed by labelling with colloidal gold particles and silver enhancements (Jackson and Cook, 1986b). In this set of experiments, the size of each replication foci remained unchanged throughout S phase, implying the total number of these sites at any given time is relatively constant. Replication factories can subsequently arrange into higher order nuclear zones or neighbourhoods, allowing for the coordination of replication and gene expression in the cell (Jackson et al., 1988; Koberna et al., 2005; Sadoni et al., 2004). To characterize these functional sites within a nucleus, simultaneous immunolabelling of HeLa cell proteins was performed with indirect immunouorescence. The proteins labelled were heterochromatin protein 1 gamma (HP1g), nascent transcript sites (TS), RNA polymerase II sites (pol II), PCNA and 4,6-diamidino-2-phenylindole (Wei et al., 1998). These components have been identied as important to the replication process and were found localized in the specic sites. Using this type of procedure, the researchers were able to show that certain regions in the nucleus are organized into specic zones where the close association of both functional sites of replication, and proteins mediating the processes allow for proper replication (Wei et al., 1998). Using the same technique and analysis on two nuclear matrix-associated proteins (matrin 3 and SAF-A), it is proposed that these proteins act as a platform for the formation of these replication zones and may potentially be directly involved with certain functions (Wei et al., 1998).
Summary and Future Directions
Although this article demonstrates that much is currently known about mammalian cell DNA replication, including many of the genes, proteins and subsequent protein complexes and their mechanisms, many critical questions remain unanswered. One primary question regarding DNA replication is focused on the organization of the replication proteins. Are these proteins assembled together throughout the cell cycle, or do they only come together when signalled to do so? If proteins remain in a complex
throughout DNA synthesis, what is the role of the nuclear matrix? Is one function of the nuclear matrix to support a stationary replication apparatus? In addition, replication initiation is tightly controlled through regulated formation and activation of pre-replication complexes. Not all prereplication complexes formed are destined to become actively ring origins in a given cellular context. Thus, much work in replication initiation is focused on predicting origin selection. With advancement of biochemical techniques comes the discovery of new interacting partners for known pre-replication complex components, perhaps complicating our picture of the cellular requirements for complex formation and activation. Eorts to predict the timing of origin ring are also ongoing, as many unanswered questions remain as to what makes some origins re earlier in S phase than others. These questions have lead some researchers to examine the spatial relationships of origins relative to genes, promoters or CpG islands. What role, if any, these genomic features have in determining origin ring is yet to be determined. Understanding how cell cycle signals and origin context integrate to regulate initiation will shed light onto how these processes aid in maintenance of genomic stability and prevention of disease. Owing to the end-replication problem in which terminal DNA is lost with each round of replication, telomeres evolved as noncoding DNA at the chromosome ends to act as a buer and protect the rest of the encoding genome from degradation. Telomeres help maintain the integrity of the genome by preventing end-to-end fusions of chromosomes and degradation. Below a critical threshold, the continued shortening of the telomeres induce an irreversible cell growth arrest (senescence), cell death or genomic instability. A main question to resolve is how closely telomere maintenance and DNA replication are connected. Does telomerase extension of telomeres use similar players as in DNA replication (e.g. primase, PCNA and ligase)? The replication and regulation of telomeres is complex and is currently undergoing intense research including understanding the proteins that protect the telomere (shelterin and chromatin complex), how the enzyme telomerase extends telomeres, the timing of telomere replication, determining the last base of each DNA strand at the telomere, the impact of telomere length and cell viability and the structure of telomeres. Understanding the telomere and its regulation will aid in conquering human diseases such as those related to ageing and cancer. Experimental data has shown that the nuclear matrix is a key factor for DNA organization and regulation of the initiation of DNA synthesis at a specic DNA sequence. DNA in the earliest stages of replication are attached to the nuclear matrix, and it has been proposed that only DNA attached to the nuclear matrix is able to correctly initiate DNA synthesis using a specic replication origin sequence. Thus far, however, the only specic matrix attachment sequences veried in higher eukaryotes are ATTA and ATTTA (Martelli et al., 2002). These recognized matrix attachment consensus sequences, however, are not
7
ENCYCLOPEDIA OF LIFE SCIENCES & 2009, John Wiley & Sons, Ltd. www.els.net
DNA Replication: Mammalian
essential elements in all higher eukaryotic replication origins isolated so far. Regardless, this does not mean attachment to the nuclear matrix is not important in the regulation of DNA synthesis, because other matrix attachment regions have yet to be identied. The assumption that DNA associates with the nuclear matrix during replication has led to the theory that the replication machinery is xed to the nuclear matrix, whereas the DNA moves through these factories. This process has been observed in living HeLa cells using uorescence microscopy (Berezney, 2002). Here, it was shown that the newly synthesized DNA may rearrange locally, but does not move to another nuclear site, which correlates to DNA movement through xed replication factories. At this time, however, it remains unseen as to how the newly created DNA moves within the replication focus. Also, the micro-architecture of these foci has yet to be studied, and the issue of distribution of synthetic sites within the foci is unresolved (Berezney and Wei, 1998). Knowledge to this point indicates that the replication factories will assemble dependent on the cell cycle, initially appearing in late G1 phase, and maintained throughout S phase. Early in the S phase, these factories are approximately 100 nm in length, and each factory can duplicate 10 replicons. Data suggests that the dierent sets of replicons are activated in a type of order throughout S phase according to nuclear structure. This may lead to a large amount of regulatory control during DNA synthesis. The regulation of the activation and assembly of replication factories, however, remains undened.
References
Aguilera A and Gomez-Gonzales B (2008) Genome instability: a mechanistic view of its causes and consequences. Nature Reviews. Genetics 9: 204217. Alberts B (2003) DNA replication and recombination. Nature 421: 431435. Anachkova B, Djeliova V, Russev G et al. (2005) Nuclear matrix support of DNA replication. Journal of Cellular Biochemistry 96(5): 951961. Berezney R (2002) Regulating the mammalian genome: the role of nuclear architecture. Advances in Enzyme Regulation 42: 3952. Berezney R and Coey DS (1974) Identication of a nuclear protein matrix. Biochemical and Biophysical Research Communications 60(4): 14101417. Berezney R and Wei X (1998) The new paradigm: integrating genomic function and nuclear architecture. Journal of Cellular Biochemistry Supplement 3031: 238242. Bianchi A and Shore D (2008) How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Molecular Cell 31(2): 153165. Cadoret JC, Meisch F, Hassan-Zadeh V et al. (2008) Genomewide studies highlight indirect links between human replication origins and gene regulation. Proceedings of the National Academy of Science of the USA 105: 1583715842. Chakhparonian M and Wellinger RJ (2003) Telomere maintenance and DNA replication: how closely are these two connected? Trends in Genetics 19(8): 439446.
Cole MD and Cowling VH (2008) Transcription-independent functions of MYC: regulation of translation and DNA replication. Nature Reviews. Molecular Cell Biology 9(10): 810815. Coverly D, Marr J and Ainscough J (2005) Ciz1 promotes mammalian DNA replication. Journal of Cell Science 118: 101112. Cvetic C and Walter JC (2005) Eukaryotic origins of DNA replication: could you please be more specic? Seminars in Cell and Developmental Biology 16(3): 343353. Dijkwel PA, Wenink PW and Poddighe J (1986) Permanent attachment of replication origins to the nuclear matrix in BHKcells. Nucleic Acids Research 14(8): 32413249. Djeliova V, Russev G and Anachkova B (2001a) Dynamics of association of origins of DNA replication with the nuclear matrix during the cell cycle. Nucleic Acids Research 29(15): 31813187. Djeliova V, Russev G and Anachkova B (2001b) Distribution of DNA replication origins between matrix-attached and loop DNA in mammalian cells. Journal of Cellular Biochemistry 80(3): 353359. Forsburg S (2008) The MCM helicase: linking checkpoints to the replication fork. Biochemical Society Transactions 36: 114119. Gilson E and Geli V (2007) How telomeres are replicated. Nature Reviews. Molecular Cell Biology 8(10): 825838. Gimenes F, Takeda KI, Fiorini A, Gouveia FS and Fernandez MA (2008) Intrinsically bent DNA in replication origins and gene promoters. Genetics and Molecular Research 7(2): 549558. He DC, Nickerson JA and Penman S (1990) Core laments of the nuclear matrix. Journal of Cell Biology 110(3): 569580. Hickey RJ, Hoelz DJ and Malkas LH (2003) DNA replication: mammalian. Encyclopedia of Life Sciences DOI: 10.1038/ npg.els.0001041 http://mrw.interscience.wiley.com/emrw/ 9780470015902/els/article/a0001041/current/html?hd=All, mammalian&hd=All,dna&hd=All,replication Hirano Y, Takahashi H, Kumeta M et al. (2008) Nuclear architecture and chromatin dynamics revealed by atomic force microscopy in combination with biochemistry and cell biology. Pugers Archives of European Journal of Physiology 456(1): 139153. Jackson DA and Cook PR (1986a) Replication occurs at a nucleoskeleton. EMBO Journal 5(6): 14031410. Jackson DA and Cook PR (1986b) A cell-cycle-dependent DNA polymerase activity that replicates intact DNA in chromatin. Journal of Molecular Biology 192(1): 6576. Jackson DA, Yuan J and Cook PR (1988) A gentle method for preparing cyto- and nucleo-skeletons and associated chromatin. Journal of Cell Science 90(part 3): 365378. Koberna K, Ligasova A, Malinsky J et al. (2005) Electron microscopy of DNA replication in 3-D: evidence for similarsized replication foci throughout S-phase. Journal of Cellular Biochemistry 94(1): 126138. Lutzmann M and Mechali M (2008) MCM9 binds Cdt1 and is required for the assembly of prereplication complexes. Molecular Cell 31(2): 190200. Martelli AM, Falcieri E and Zweyer M (2002) The controversial nuclear matrix: a balanced point of view. Histology and Histopathology 17(4): 11931205. Meselson M and Stahl FW (1958) The Replication of DNA in Escherichia Coli. Proceedings of the National Academy of Sciences of the USA 44(7): 671682.
ENCYCLOPEDIA OF LIFE SCIENCES & 2009, John Wiley & Sons, Ltd. www.els.net
DNA Replication: Mammalian
Nakamura H, Morita T, Masaki S and Yoshida S (1984) Intracellular localization and metabolism of DNA polymerase alpha in human cells visualized with monoclonal antibody. Experimental Cell Research 151(1): 123133. Nakayasu H and Berezney R (1989) Mapping replicational sites in the eucaryotic cell nucleus. Journal of Cell Biology 108(1): 111. Ohta S, Tatsumi Y, Fujita M, Tsurimoto T and Obuse C (2003) The ORC1 cycle in human cells: II. Dynamic changes in the human ORC complex during the cell cycle. Journal of Biological Chemistry 278(42): 4153541540. Olovnikov AM (1973) A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological signicance of the phenomenon. Journal of Theoretical Biology 41(1): 181190. Philimonenko AA, Jackson DA, Hodny Z et al. (2004) Dynamics of DNA replication: an ultrastructural study. Journal of Structural Biology 148(3): 279289. Radichev I, Parashkevova A and Anachkova B (2005) Initiation of DNA replication at a nuclear matrix-attached chromatin fraction. Journal of Cellular Physiology 203(1): 7177. Rossi ML, Purohit V, Brandt PD and Bambara RA (2006) Lagging strand replication proteins in genome stability and DNA repair. Chemical Reviews 106: 453473. Sadoni N, Cardoso MC and Stelzer EH, Leonhardt H and Zink D (2004) Stable chromosomal units determine the spatial and temporal organization of DNA replication. Journal of Cell Science 117(part 22): 53535365.
Sfeir AJ, Shay JW and Wright WE (2005) Fine-tuning the chromosome ends: the last base of human telomeres. Cell Cycle 4: 14671470. Takeda DY and Dutta A (2005) DNA replication and progression through S phase. Oncogene 24(17): 28272843. Trujillo KM and Osley MA (2008) INO80 meets a fork in the road. Nature Structural and Molecular Biology 15(4): 332334. Walther AP and Wold MS (2001) Eukaryotic replication fork. Encyclopedia of Life Sciences DOI: 10.1038/npg.els.0001050 http://mrw.interscience.wiley.com/emrw/9780470015902/els/ article/a0001050/current/html?hd=All,walther&hd=All,wold Wan KM, Nickerson JA, Krockmalnic G and Penman S (1999) The nuclear matrix prepared by amine modication. Proceedings of the National Academy of Sciences of the USA 96(3): 933938. Watson JD and Crick FH (1953) Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171(4356): 737738. Wei X, Samarabandu J, Devdhar RS et al. (1998) Segregation of transcription and replication sites into higher order domains. Science 281(5382): 15021506.
Further Reading
Bielinsky AK (2003) Replication origins: why do we need so many? Cell Cycle (JulyAugust) 2(4): 307309.
ENCYCLOPEDIA OF LIFE SCIENCES & 2009, John Wiley & Sons, Ltd. www.els.net
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- CRISPR-Cas9 Structures and Mechanisms: FurtherDocument27 pagesCRISPR-Cas9 Structures and Mechanisms: FurtherКристијан ЈунузовскиNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- CH 7 Nucleic AcidsDocument8 pagesCH 7 Nucleic AcidsInes Ait Si SelmiNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- 2020 - Article - 18180 11Document13 pages2020 - Article - 18180 11PabloNo ratings yet
- Protein Synthesis EssayDocument5 pagesProtein Synthesis Essaymarybrownarlington100% (2)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Unit 1 Amplification TechniqueDocument9 pagesUnit 1 Amplification TechniqueShin BoyceNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Intro To Biohacking: or "How I Learned To Stop Worrying and Love The Zombie Apocalypse"Document31 pagesIntro To Biohacking: or "How I Learned To Stop Worrying and Love The Zombie Apocalypse"rasromeoNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- CRISPR/Cas9-based Efficient Genome Editing in Clostridium Ljungdahlii, An Autotrophic Gas-Fermenting BacteriumDocument32 pagesCRISPR/Cas9-based Efficient Genome Editing in Clostridium Ljungdahlii, An Autotrophic Gas-Fermenting BacteriumShampa SenNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- 60 Assertion Reason MCQs by DR NK Sharma Molecular Basis of InheritanceDocument8 pages60 Assertion Reason MCQs by DR NK Sharma Molecular Basis of InheritanceDiNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Eukaryotic DNA Polymerases: Sue Cotterill, Stephen KearseyDocument6 pagesEukaryotic DNA Polymerases: Sue Cotterill, Stephen KearseyOphy FirmansyahNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Ogbonnaya AkparaDocument3 pagesOgbonnaya AkparaOgbonnaya Jr AkparaNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Expression in Escherichia Coli of A Chemically Synthesized Gene For The Hormone SomatostatinDocument2 pagesExpression in Escherichia Coli of A Chemically Synthesized Gene For The Hormone SomatostatinDaniel JmzNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- 6.5 DNA Strucuture and Replication OriginalDocument4 pages6.5 DNA Strucuture and Replication OriginalSteelcrow14No ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Transcription and Translation Simulation WorksheetDocument4 pagesTranscription and Translation Simulation WorksheetDonna NNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- BIO 216-Molecular Biology-Sec1-Muhammad Tariq-Shaper Mirza-Syed Shahzad Ul HussanDocument3 pagesBIO 216-Molecular Biology-Sec1-Muhammad Tariq-Shaper Mirza-Syed Shahzad Ul HussanAnonymous sF8ZuiGNo ratings yet
- Discussion Assignment Unit 4 Biochemistry Chem 3212Document2 pagesDiscussion Assignment Unit 4 Biochemistry Chem 3212Kingsley OsujiNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- BTN 315 Exam Prep ch1 4Document3 pagesBTN 315 Exam Prep ch1 4Corbyn JasonNo ratings yet
- CLONING EXERCISEsDocument4 pagesCLONING EXERCISEsIronic SunNo ratings yet
- Prokariotik and Eukariotik GenesDocument68 pagesProkariotik and Eukariotik Genesarief270490No ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- NucleusDocument10 pagesNucleusAyesha Saleem100% (1)
- Stahl and Meselson'S Work On Semiconservative Nature of Dna ReplicationDocument12 pagesStahl and Meselson'S Work On Semiconservative Nature of Dna ReplicationKima MadNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- SSC Gr8 Biotech Q4 Module 1 WK 1 - v.01-CC-released-09May2021Document22 pagesSSC Gr8 Biotech Q4 Module 1 WK 1 - v.01-CC-released-09May2021Ivy JeanneNo ratings yet
- 357 - Cell-Biology Physiology) DNA Transcription001Document12 pages357 - Cell-Biology Physiology) DNA Transcription001SodysserNo ratings yet
- Primer Annealing TemperatureDocument1 pagePrimer Annealing TemperatureManu Mallahalli ShanthappaNo ratings yet
- Recognition, Signaling, and Repair of DNA Double-Strand Breaks Produced by Ionizing Radiation in Mammalian Cells - The Molecular ChoreographyDocument89 pagesRecognition, Signaling, and Repair of DNA Double-Strand Breaks Produced by Ionizing Radiation in Mammalian Cells - The Molecular ChoreographyMaria ClaraNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- 2022 Nucleic Acid and Gene Expression Problem Set (Teacher)Document28 pages2022 Nucleic Acid and Gene Expression Problem Set (Teacher)Sundaravadivel Prabhav (Njc)No ratings yet
- 4.3 Genetic Diversity Via Mutation QP PDFDocument11 pages4.3 Genetic Diversity Via Mutation QP PDFMuffarrahNo ratings yet
- Noordermeer 2022Document102 pagesNoordermeer 2022Rin ChanNo ratings yet
- SBT1043 Biotechnology Concepts and Techniques Test 1Document7 pagesSBT1043 Biotechnology Concepts and Techniques Test 1Alia HanisaNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Chapter 13 Test A RNA and Protein Synthesis ANSWERS PDFDocument6 pagesChapter 13 Test A RNA and Protein Synthesis ANSWERS PDFxspiiirONo ratings yet
- Dogma Central GenéticaDocument6 pagesDogma Central GenéticaDIEGO JOSÉ MACHADO ARIASNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)