Professional Documents
Culture Documents
Use of Nano Materials in Water Purification
Uploaded by
Charudatta GalandeOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Use of Nano Materials in Water Purification
Uploaded by
Charudatta GalandeCopyright:
Available Formats
Use of nanomaterials in water purification
The recent earthquake in Haiti has focused worldwide attention on the need for improved water purification materials and systems. Numerous individuals, religious charities, non-governmental organizations, and private companies have sent water purifications systems to Haiti in recent months in order to stem the spread of waterborne diseases. This recent tragedy has placed a spotlight on the ongoing problem of inadequate access to safe water in developing countries. The United Nations estimates that 1.1 billion people, or eighteen per cent of the world population, cannot obtain safe water at this time1. In developing countries, waterborne diseases such as cholera, dysentery, enteric fever, and hepatitis A are quite common2. Endemic diarrheal diseases place individuals, particularly children, at risk of arrested growth, malnutrition, and neurological conditions. The World Health Organization states that 1.6 million individuals, mostly young children, die from diarrheal diseases each year1.
Roger Narayan Joint Department of Biomedical Engineering, NC State University and UNC Chapel Hill E-mail: roger_narayan@msn.com
Improving water quality provides social and economic benefits to developing countries. For example, chlorination, filtration, solar disinfection, or combined flocculation and chlorination can yield benefits of $5 to $140 for each $1 that is invested1. In developing countries, large-scale municipal water treatment systems face problems such as inadequate maintenance, intermittent delivery, contamination with microorganisms, and lack of chlorination3-6. The high cost of water transportation and the high cost of
constructing centralized water systems also have limited the use of centralized water purification4,5. Disinfecting water after collection, which is commonly referred to as point-of-use disinfection, enables individuals to improve water obtained from unsafe sources6-9. Low-cost point-of-use technologies are being developed to convert water from untreated sources such as springs, wells, community taps, and rivers7. At this time, chlorination, flocculation, boiling, and filtration are the most commonly used point-
44
JUNE 2010 | VOLUME 13 | NUMBER 6
ISSN:1369 7021 Elsevier Ltd 2010
Use of nanomaterials in water purification
APPLICATION
Fig.1 Plan-view scanning electron micrograph obtained from the large pore side of a zinc oxide-coated 20 nm pore size nanoporous alumina membrane.
of-use water purification technologies. 3040% reductions in diarrheal disease have been attributed to point-of-use treatments10. In addition, point-of-use water treatment involves lower energy costs and provides more straightforward control over water treatment than conventional methods. In recent years, the use of nanostructured materials in point-ofuse water purification devices has been considered11. Nanostructured materials exhibit several advantages over conventional microstructured materials for water purification, including larger relative surface areas12. These chlorine-free water purification methods are of particular interest since carcinogenic disinfection byproducts may be formed when components of natural water interact with chloramines or chlorine13. For example, Van de Bruggen et al. have described using nanoporous membranes for removal of arsenic, bacteria, organic
material, nitrates, salinity, and viruses from groundwater and surface water14. Srivastava et al. demonstrated the use of membranes containing radially aligned carbon nanotube walls for removal of viruses and bacteria, including Poliovirus sabin 1, Escherichia coli, and Staphylococus aureus15. Thermal processes may be used to regenerate carbon nanotube membranes16. Micro-organisms may form biofilms on the surfaces of water purification membranes. These biofilms decrease membrane permeability and increase water purification costs17-19. In addition, some microorganisms may release substances that degrade water quality, such as metabolic products and biological toxins20,21. Conventional methods for preventing the formation of microbial biofilms involve treating membranes with biocidal agents; however, biocidal agents may not be effective in eliminating rapidly growing
Fig. 2 Cross-sectional scanning electron micrograph obtained after fracturing a 60 m thick nanoporous alumina membrane.
JUNE 2010 | VOLUME 13 | NUMBER 6
45
APPLICATION
Atomic layer deposition of nanoporous biomaterials
bacteria22,23. In addition, biocidal agents may destroy the surfaces of water purification membranes23. Several investigators have described fabrication of nanostructured ceramic membranes containing zinc oxide and the anatase phase of titania24-26. Zinc oxide and the anatase phase of titania have been shown to photocatalytically degrade pollutants as well as prevent growth of microorganisms24-28. Unlike conventional polymeric membranes, ceramic membranes do not undergo degradation when exposed to heat or ultraviolet light25. Functionalization of nanoporous membranes with photocatalytic titania coatings has attracted significant interest over the past few years. When irradiated by an ultraviolet A light source (e.g., solar energy), titania is able to degrade organic contaminants as well as destroy microorganisms24,29. For example, Zhang et al. demonstrated that silica/ titania nanotube composite membranes on porous alumina support membranes removed Direct Black 168 dye by means of membrane separation and photocatalysis25. In another study, Ma et al. used a solgel method in order to coat silicon-doped titania layers on commercial alumina membranes11. These membranes demonstrated removal and photocatalytic degradation of a model pollutant (Reactive Red ED-2B). Zhang et al. grafted anatase titania nanotubes within the channels of alumina microfiltration membranes by means of a liquid-phase deposition method30. Membranes with titania nanotube inner diameters between 5 nm and 100 nm were obtained using this technique. These titania nanotube membranes demonstrated photocatalytic degradation of humic acid as well as reductions in membrane biofouling. Antimicrobial coatings and photocatalytic coatings have also been deposited on nanoporous membranes using atomic layer deposition26,31. For example, atomic layer deposition was used to deposit anatase titania coatings on nanoporous alumina membranes, which exhibit straight
pores, high pore densities, and small pore sizes. Scanning electron microscopy of cross-sectional samples revealed that titania nanocrystals extended to the middle of the 60 mm thick nanopores. Titania-coated 20 nm pore size nanoporous alumina membranes that were exposed to ultraviolet light demonstrated activity against Escherichia coli and Staphylococus aureus bacteria. Atomic layer deposition may be useful in creating membranes with extremely small pore sizes for preventing penetration of viruses. Nanoporous membranes with biocidal properties as well as other nanostructured materials may be useful in the development of point-of-use water purification systems for developing countries and emergency situations. It should be noted that nanostructured material-based water purification technologies are also being incorporated within centralized water systems in developed countries32. For example, use of nanofiltration in a large distribution system was shown to reduce the amount of microorganisms and organic material33. In addition, fouling-resistant membranes may be used in distributed optimal technology networks; these networks are being considered as an alternative to centralized water treatment facilities34. Efforts are also underway to develop nanostructured materials for water purification with other functionalities, including removal of radionuclides and desalination35,36. For these efforts to have a significant impact, it will be necessary to reduce the processing costs for nanostructured water purification materials so that they are similar to those for conventional ceramic and polymeric water purification materials. In addition, comparisons between nanostructured water purification materials and their conventional counterparts with regard to effectiveness over extended periods of time are also needed.
REFERENCES
1. WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. Water for Life: Making It Happen. WHO Press, Geneva, 2005. 2. Sobsey, M. D., et al., Environ Sci Technol (2008) 42, 4261. 3. Varghese, A., A comparative risk approach to assessing point-of-use water treatment systems in developing countries. In Comparative Risk Assessment and Environmental Decision Making, Linkov, I., Ramadan A. B., (eds.), NATO Science Series IV Earth and Environmental Sciences, Rome, (2004), 38, 99. 4. Gleick, P. H., Science (2003) 302,1524. 5. Haas, C. N., J Am Water Works Assoc (2000) 92, 72. 6. Mintz, E., et al., J Amer Med Assoc (1995) 273, 948. 7. Colindres, R. E., et al., J Water Health (2007) 5, 367. 8. Elimelech, M., J Water Supply Res Technol (2006) 55, 3. 9. Quick, R. E., et al., Epidemiol Infect (1999) 122, 83. 10. Sobsey, M. D., et al., Environ Sci Technol (1008) 42, 4261. 11. Ma, N., et al., J Membr Sci (2009) 335, 58. 12. Savage, N., and Diallo, M. S., J Nanopart Res (2005) 7, 331. 13. Krasner, S. W., et al., Environ Sci Technol (2006) 40, 7175. 14. Van der Bruggen, B., et al., Environ Poll (2003) 122, 435. 15. Srivastava, A., et al., Nature Mater (2004) 3, 610. 16. Brady-Estevez, S., et al., Small (2008) 4, 481.
17. Hilal, N., et al., Desalin (2004) 167, 293. 18. Kochkodan, V., et al., Desalin (2008) 220, 380. 19. Liu, C. X., et al., J Membr Sci (2010) 346, 121. 20. Park, N., et al., J Membr Sci (2005) 258, 43. 21. Upadhyayula, V. K. K., et al., Sci Total Environ (2009) 408, 1. 22. Flemming, H. C., et al., Desalin (1997) 113, 215. 23. Kim, D., et al., Desalin (2009) 238, 43. 24. Li, Q., et al., Water Res (2008) 42, 4591. 25. Zhang, H. M., et al., Environ Sci Technol (2006) 40, 6104. 26. Narayan, R. J., et al., Phil Trans Royal Soc A (2010) 368, in press. 27. Aal, A. A., et al., Mater Sci Eng C (2009) 29, 831. 28. Gittard, S. D., et al., Appl Surf Sci (200) 255, 5806. 29. Danion, A., et al., Appl Catal B (2004) 52, 213. 30. Zhang, X. W., et al., Appl Catal B (2008) 84, 262. 31. Narayan, R. J., et al., JOM (2009) 61, 12. 32. Smith, A., Filtr Sep (2006) 43, 32. 33. Peltier, S., et al., Water Sci Technol Water Supply (2003) 3,193. 34. Weber Jr., W. J., Water Sci Technol (2002) 46, 241. 35. Favre-Reguillon, A., et al., Ind Eng Chem Res (2003) 42, 5900. 36. Mohsen, M. S., et al., Desalin (2003) 157, 167.
46
JUNE 2010 | VOLUME 13 | NUMBER 6
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- 3.5 Standard Operations Research Models: Mathematical Modify ModelDocument2 pages3.5 Standard Operations Research Models: Mathematical Modify ModelAnonymous IjhB0kuFNo ratings yet
- Itil Intermediate Capability Stream:: Operational Support and Analysis (Osa) CertificateDocument33 pagesItil Intermediate Capability Stream:: Operational Support and Analysis (Osa) CertificateNitinNo ratings yet
- Science of Getting RichDocument150 pagesScience of Getting RichTony Okpere Jr.100% (4)
- A Ride in The Safari ParkDocument8 pagesA Ride in The Safari ParkSyahida Saharin AbdullahNo ratings yet
- 5.occlusal Risk Factors Associated With Temporomandibular Disorders in Young Adults With Normal OcclusionsDocument5 pages5.occlusal Risk Factors Associated With Temporomandibular Disorders in Young Adults With Normal Occlusionsthiên lữNo ratings yet
- Learning Chara Dasa K N RaoDocument34 pagesLearning Chara Dasa K N RaoVikram Kumar100% (3)
- Cursive Writing LP MembersDocument7 pagesCursive Writing LP MembersAng gnalikaD KomentoNo ratings yet
- P1 2012 Dec QDocument6 pagesP1 2012 Dec QBilal AliNo ratings yet
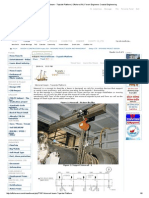
- Monorail Beam - Topside Platform - OffshoreDocument6 pagesMonorail Beam - Topside Platform - OffshoreBolarinwaNo ratings yet
- HDMI Cable Modeling and Analysis With TDR DataDocument9 pagesHDMI Cable Modeling and Analysis With TDR DataAmmar AbdullahNo ratings yet
- Simpson S RuleDocument13 pagesSimpson S RuleJunaid AhmedNo ratings yet
- The Final Revesion of My Paper in Wulfenia Journal PDFDocument22 pagesThe Final Revesion of My Paper in Wulfenia Journal PDFAli Abdul-RahmanNo ratings yet
- Servo Motor Control Application On A Local Interconnect Network (LIN)Document31 pagesServo Motor Control Application On A Local Interconnect Network (LIN)Diego CadoreNo ratings yet
- Median10.05 Qu25Z MidtermDocument3 pagesMedian10.05 Qu25Z MidtermLuwalhati TomilasNo ratings yet
- Chios Reiki Attunement ManualDocument12 pagesChios Reiki Attunement Manualkeithmac100% (1)
- Bihar SI Mains Syllabus-024b287317594Document3 pagesBihar SI Mains Syllabus-024b287317594Aryan KhanNo ratings yet
- Operational Categorization STDocument3 pagesOperational Categorization STFalcon Peregrine100% (1)
- Zero To HeroDocument253 pagesZero To Herodidine50% (2)
- Cold Pilgering: Presented by:-TARANG MEHTA (13103197)Document20 pagesCold Pilgering: Presented by:-TARANG MEHTA (13103197)Tarang MehtaNo ratings yet
- Notebook Three: Leadership Begins With An AttitudeDocument19 pagesNotebook Three: Leadership Begins With An AttitudeJessie James YapaoNo ratings yet
- Six Levels of Linguistic AnalysisDocument12 pagesSix Levels of Linguistic AnalysisRoshio Tsuyu Tejido67% (3)
- 15.1 Composition of MatterDocument23 pages15.1 Composition of MatterKunal GaikwadNo ratings yet
- Group 16 CRM Project On Airtel 16Document15 pagesGroup 16 CRM Project On Airtel 16Ravi DahiyaNo ratings yet
- Ctenocephalides Felis Felis vs. Ctenocephalides Canis (Siphonaptera: Pulicidae) : Some Issues in Correctly Identify These SpeciesDocument11 pagesCtenocephalides Felis Felis vs. Ctenocephalides Canis (Siphonaptera: Pulicidae) : Some Issues in Correctly Identify These SpeciesstfnhmwnNo ratings yet
- Robotech - TimelineDocument9 pagesRobotech - TimelineRobert BestNo ratings yet
- Ethical Theories AssignmentDocument6 pagesEthical Theories AssignmentHarvard TutorNo ratings yet
- Human Resources Management Practices in Modern WorldDocument7 pagesHuman Resources Management Practices in Modern WorldMaheshkumar MohiteNo ratings yet
- The Falklands War: Causes and LessonsDocument13 pagesThe Falklands War: Causes and Lessonsgtrazor100% (1)
- Micro Lab Act 2Document3 pagesMicro Lab Act 2RHINROMENo ratings yet
- Chapter #06 GravitationDocument13 pagesChapter #06 GravitationSIR USMAN KHANNo ratings yet