Professional Documents
Culture Documents
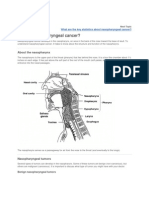
Paratesticular Sarcomas
Uploaded by
benandlouyouOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Paratesticular Sarcomas
Uploaded by
benandlouyouCopyright:
Available Formats
Original Paper
Urologia
Internationalis
Urol Int 2009;82:448452 DOI: 10.1159/000218536
Received: November 14, 2007 Accepted after revision: April 28, 2008
Paratesticular Sarcomas in Brazil
Fernando Korkes Marlia G. Castro Maria F. Amary Roni C. Fernandes
Free Author Copy for personal use only
Godoy
ANY DISTRIBUTION OF THIS ARTICLE WITHOUT WRITTEN CONSENT FROM S. KARGER Frederico R. Romero Guilherme AG, BASEL IS A VIOLATION Marjo D. Perez OF THE COPYRIGHT.
Written permission to distribDivision of Urology and Department of Pathology, Medical School of Santa Casa of So Paulo, So Paulo, Brazil ute the PDF will be granted against payment of a permission fee, which is based on the number of accesses required. Please contact permission@karger.ch
Key Words Sarcoma Testis Scrotum Paratesticular
Abstract Purpose: Paratesticular sarcomas are rare and frequently reported as isolated case reports. Studies evaluating the relative frequency of the paratesticular sarcomas are limited, and to the best of our knowledge, this is the first study of paratesticular sarcomas in the Brazilian population. Patients and Methods: Medical records of all patients undergoing treatment for paratesticular sarcomas between 1993 and 2006 were retrieved from the archives of our institution. Results: Complete data from 12 patients (39 8 23 years, range 1378) with paratesticular sarcomas were available, which represented 6.7% of all orchiectomies performed for testicular malignancies in the same period. At the time of diagnosis, 3 patients had retroperitoneal spread of the disease, all of which had elevated serum lactic dehydrogenase levels. The remaining 9 patients had normal serum markers. There were 6 rhabdomyosarcomas, 4 leiomyosarcomas, 1 liposarcoma and 1 undifferentiated sarcoma. Median follow-up was 31.4 months. Primary surgical excision by inguinal approach was performed in all cases (radical orchiectomy in 10 and preservation of the testis in 2). Retroperitoneal lymph node dissection was performed in 3 patients and excision of the hemiscrotum in 1. Eight patients received adjuvant chemotherapy. Mean overall survival time was 27.8 8 6.2 months after orchiectomy. Conclusion: Patients with paratesticular sarcomas are at high risk of disease progression, and systemic re-
lapse remains a significant problem, determining poor prognosis. The high risk of local recurrence demands long-term follow-up, and intraoperative frozen section analysis might be of benefit. Elevated lactic dehydrogenase might also be a marker of retroperitoneal disease and poor prognosis. Improvement in survival requires effectivepersonal use only Free Author Copy for systemic adjuvant therapy.DISTRIBUTION OF THIS ARTICLE WITHOUT WRITTEN 2009 S. Karger AG,KARGER AG, BASEL IS A V Copyright ANY CONSENT FROM S. Basel
Written permission to distribute the PDF will be granted against payment of a permission fee, which is
Introduction
Paratesticular sarcomas are rare and frequently reported as isolated case reports or small series of patients [13]. Optimal local and systemic treatment of these tumors remains controversial, since information is based on small retrospective studies with limited follow-up. Recent advances in the pathological classification make generalization regarding treatment and prognosis even more difficult. There are few cases reported in the literature with intermediate and long-term follow-up. For paratesticular rhabdomyosarcomas, large studies have been reported by pediatric international multi-institutional studies [46]. For leiomyosarcomas and liposarcomas, however, there are even fewer reports [7, 8]. As a further matter, studies evaluating the relative frequency of the paratesticular sarcomas are limited, and to the best of our knowledge, this is the first study of paratesticular sarcomas in the Brazilian population.
Fernando Korkes Rua Pirapora, 167 04008-060 So Paulo SP (Brazil) Tel. +55 11 3884 2244, Fax +55 11 3884 2233 E-Mail fkorkes@terra.com.br
Fax +41 61 306 12 34 E-Mail karger@karger.ch www.karger.com
2009 S. Karger AG, Basel 00421138/09/08240448$26.00/0 Accessible online at: www.karger.com/uin
Table 1. Clinicopathological features of 12 patients with paratesticular sarcomas
Age years 24 56 55 21 16 17 13 19 78 74 57 43
Initial presentation/time right scrotal mass/4 months
Histology/grade rhabdomyosarcoma/high
Surgery orchiectomy orchiectomy orchiectomy orchiectomy orchiectomy orchiectomy orchiectomy orchiectomy orchiectomy orchiectomy nodulectomy nodulectomy
Adjuvant therapy
Local Distant failure failure yes yes yes no no yes no yes no no no no
right scrotal mass/18 months leiomyosarcoma/I left scrotal mass/2 months undifferentiated sarcoma/ high right scrotal mass/5 months rhabdomyosarcoma/high left scrotal mass/3 months rhabdomyosarcoma/high left scrotal mass/8 months rhabdomyosarcoma/high right scrotal mass/9 months right scrotal mass/1 month left scrotal mass/8 months right scrotal mass/4 months left scrotal mass/6 months left scrotal mass/3 months rhabdomyosarcoma/high rhabdomyosarcoma/high leiomyosarcoma/2 liposarcoma/1 leiomyosarcoma/2 leiomyosarcoma/high
chemotherapy + retroperitoneal no lymph node dissection no chemotherapy + excision yes hemiscrotum chemotherapy chemotherapy no chemotherapy + retroperitoneal yes lymph node dissection chemotherapy + retroperitoneal no lymph node dissection chemotherapy no no no no no excision of hemiscrotum no no no
Patients and Methods
Medical records of all men treated for paratesticular sarcomas between 1993 and 2006 were retrieved from the archives of our institution. Two histopathologists (M.F.A. and M.G.C.) reviewed all slides and immunohistochemical panels to confirm the diagnoses. Clinical data, histopathological findings, treatment modalities and oncological outcomes were reviewed. Men were treated with a multimodal approach, including surgery, radiotherapy and chemotherapy. Survival estimates were computed using the Kaplan-Meier method, and survivals were compared using the log-rank test.
levels (range 445563 ng/dl). The remaining 9 (75%) patients had normal serum markers. Tumor Characteristics Tumor characteristics are shown in table 1. Mean 8 SD tumor weight was 127 8 75 g (range 15242). Surgical margins were negative in 9 (75%) patients. Median follow-up was 31.4 months (range 660). Only 2 patients were lost to follow-up. Leiomyosarcomas comprised one third of the paratesticular sarcomas. Mean 8 SD patient age was 58.3 81 4.8 (range 4278), similar to other series [9]. Mortality rate was 50%, with both cases presenting pulmonary metastasis. Two patients underwent nodulectomy, and both of them had no evidence of disease at a follow-up of 8 and 48 months. Liposarcomas represented 8.3% of paratesticular sarcomas. Other series have shown a prevalence rate that ranges from 3 to 50% [1012]. Most paratesticular liposarcomas are well differentiated and slow growing, but tend to recur if incompletely excised. Average age ranges within the 6th and 7th decades of life, and the present man was slightly older (74 years) [8, 10]. As is commonly seen in patients with this rare tumor, he presented with local disease and showed a good prognosis. For these cases, retroperitoneal lymphadenectomy does not seem to offer any additional therapeutic benefit and the role of chemotherapy is not well defined. Regardless of initial
Urol Int 2009;82:448452
Results
Prevalence Complete data from 12 men with paratesticular sarcomas were available, which represented 6.7% of all orchiectomies performed for testicular malignancies during that period at our institution. Patient Demographics and Clinical Characteristics Median 8 SE age at diagnosis was 39 8 23 years (range 1378). All men presented with painless scrotal swelling, and mean 8 SD history time was 5.7 8 4.6 months (range 118). At the time of diagnosis, 3 patients (25%) had retroperitoneal spread of the disease, all of which had elevated serum lactic dehydrogenase (LDH)
Paratesticular Sarcomas in Brazil
449
Color version available online
Fig. 1. Macroscopic aspect of paratesticular rhabdomyosarcoma
associated with hydrocele and testis compression.
Fig. 2. Histology of paratesticular rhabdomyosarcoma: interlac-
ing fascicles of atypical spindle cells. Hematoxylin and eosin. !25.
therapy, the risk of local recurrence always requires longterm follow-up [10]. Rhabdomyosarcoma is the most frequent malignant tumor of paratesticular origin [13]. It is classified as embryonal, alveolar, pleomorphic and mixed. Embryonal form is most frequent in childhood and has the best prognosis (fig. 1, 2). Alveolar rhabdomyosarcoma occurs mainly in adolescents and young adults. The pleomorphic subtype occurs in adults and bears the worst prognosis [13]. At our institution, rhabdomyosarcomas represented 50% of all paratesticular sarcomas. It was found mainly in young men, with a mean 8 SD age of 18.3 8 3.9 years (range 1324). The most common pathological variant was the embryonal rhabdomyosarcoma (83%, n = 5), followed by the pleomorphic subtype (17%, n = 1). Three of these men initially presented with metastatic disease and died after a mean period of 10 months after surgery, despite chemotherapy. Two are alive and cured, and 1 demonstrated retroperitoneal disease after 10 months of surgery. He is currently undergoing adjuvant chemotherapy. Treatment Modalities Primary surgical excision by the inguinal approach was performed in all cases. In 2 cases (16.7%), complete excision of the tumor with preservation of the testis was
450
Urol Int 2009;82:448452
possible. Radical orchiectomy with high ligation of the spermatic cord was required in 10 patients (83.3%). Retroperitoneal lymph node dissection for secondary tumor spread was performed in 3 patients, and excision of the hemiscrotum in 1, for local invasion. Eight patients (66.7%) received adjuvant chemotherapy. There were 2 local failures, and 6 patients developed distant metastases, including the lungs, liver, bones, peritoneum and lymph nodes. Oncological Outcomes Median follow-up was 31.4 months (range 660 months) and 2 patients were lost to follow-up. Mean 8 SD overall survival time was 27.8 8 6.2 months after orchiectomy (fig. 3). Six patients (50%) died at a mean follow-up of 22 months after surgery (range 448): 2 with leiomyosarcoma, 3 with rhabdomyosarcoma and 1 with undifferentiated sarcoma. For rhabdomyosarcoma, mean 8 SD overall survival was 11.4 8 2.1 months and for leiomyosarcoma it was 44.0 8 4.6 months. There was no difference in diseasespecific survival when comparing patients with rhabdomyosarcoma and leiomyosarcoma (p = 0.24).
Korkes/Castro/Romero/Godoy/Amary/ Fernandes/Perez
Color version available online
Survival distribution function
1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 10 20 30 40 Months 50 60 70
Fig. 3. Kaplan-Meier estimation of disease-specific survival of 12
patients with paratesticular sarcoma.
Discussion
Soft tissue sarcomas are aggressive tumors with a tendency towards local recurrence and systemic dissemination. Paratesticular are rare neoplasms that can arise from the epididymis, mesenchymal layers surrounding the testis and its true appendages or spermatic cord. It is often difficult, however, to localize the precise origin of these tumors [14] (fig. 1). Our study has some important findings. First, even in large institutions it is difficult to accumulate sufficient cases to document the natural history of paratesticular sarcomas and to draw conclusions about treatment results. At our institution, they represented 6.7% of orchiectomies performed for testicular cancer over a 13-year period. Furthermore, as this study is the first report of a Brazilian series, it might bring additional original data, since these neoplasms may vary according to environmental characteristics and changes [15]. When comparing it to the largest series available [12], our series had a higher frequency of leiomyosarcomas (33.3 vs. 19.0%), higher frequency of rhabdomyosarcomas (50 vs. 19%) and lower frequency of liposarcomas (8.3 vs. 44%). In our series, disease-specific mortality was higher, which can be explained by the lower frequency of better prognosis tumors such as liposarcomas [12]. Second, we could evaluate outcomes for different surgical approaches, as detailed in the Results section. Even though radical surgery is the first-line treatment for paratesticular sarcomas, 2 men with leiomysarcomas were
Paratesticular Sarcomas in Brazil
treated with nodulectomy and intraoperative frozen section analysis. This approach had been previously reported for paratesticular liposarcomas [16]. However, since conservative surgery is an exception for these tumors, little data is available [17]. These men do not show any sign of recurrence after 8 and 48 months of follow-up. Additionally, if frozen section analysis had been done in all surgeries, maybe we would not have as much as 25% positive margins. Even though it is not the scope of the present study to prove this statement, in our opinion, frozen section analysis should be considered a standard instrument when paratesticular sarcoma is suspected. If extensive resection is required to achieve negative histological margins, it should be undertaken and reconstructive techniques might be of value [18]. Additionally, adjuvant radiotherapy may also be considered to reduce local recurrence [18]. Third, LDH levels were shown to be a marker of retroperitoneal disease in the present group of men. None of the men with retroperitoneal metastasis had normal LDH levels, and all men with elevated LDH levels showed retroperitoneal evidence of disease at CT scan. LDH was also a poor prognostic marker in the present series, as all patients with elevated LDH at presentation died from disease after a mean period of 10 months. This finding is similar to that reported for soft tissue sarcomas [19]. Our study has several limitations. First, the number of patients is low and they were retrospectively evaluated, which limits the power for further conclusions. Furthermore, 2 patients were lost to follow-up. However, the strength of our study is to describe a series of men from a different region, since the few series available included men from North America and Europe [2, 12, 20]. The main goals in the treatment of soft tissue sarcomas are to perform wide en bloc excisions of all contaminated tissues and to combine adjuvant therapies. As previously reported, a policy of wide repeated resection of tissues at risk including excision of the hemiscrotum, when appropriate, can benefit the patients and lower the toxicity of high-dose radiation to the hemiscrotum and groin [21]. Retroperitoneal lymph node dissection can be used for both staging and treatment of paratesticular sarcomas [13]. Different from germ cell tumors, however, treatment guidelines for paratesticular sarcomas are not well established, since dissemination occurs by both hematogenous and lymphatic routes. Adjuvant loco-regional radiotherapy reduces the risk of local recurrence, and doxorubicin-based chemotherapy may improve time to local and distant failure and increase overall survival. The role of radio- and chemotherapy in paratesticular sarcomas, however, is not fully established [22].
Urol Int 2009;82:448452
Color version available online
451
Conclusions
Patients with paratesticular sarcomas are at high risk of disease progression and systemic relapse remains a significant problem, determining poor prognosis. The high
risk of local recurrence demands long-term follow-up, and intraoperative frozen section analysis might be of benefit. Elevated LDH might also be a marker of retroperitoneal disease and poor prognosis. Improvement in survival requires effective systemic adjuvant therapy.
References
1 Radouane B, El Fenni J, Chaouir S, et al: Paratesticular rhabdomyosarcoma. A case report. J Radiol 2004;85:779781. 2 Mondaini N, Palli D, Saieva C, et al: Clinical characteristics and overall survival in genitourinary sarcomas treated with curative intent: a multicenter study. Eur Urol 2005;47: 468 473. 3 Mora Nadal JI, Ponce Campuzano A, Llopis Manzanera J, et al: Paratesticular rhabdomyosarcoma. Actas Urol Esp 2004;28:245 248. 4 Stewart RJ, Martelli H, Oberlin O, et al: Treatment of children with nonmetastatic paratesticular rhabdomyosarcoma: results of the Malignant Mesenchymal Tumors studies (MMT 84 and MMT 89) of the International Society of Pediatric Oncology. J Clin Oncol 2003;21:793798. 5 Ferrari A, Bisogno G, Casanova M, et al: Paratesticular rhabdomyosarcoma: report from the Italian and German Cooperative Group. J Clin Oncol 2002;20:449 455. 6 Stevens MC, Rey A, Bouvet N, et al: Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Paediatric Oncology SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol 2005;23:26182628. 7 Fisher C, Goldblum JR, Epstein JI, et al: Leiomyosarcoma of the paratesticular region: a clinicopathologic study. Am J Surg Pathol 2001;25:11431149. 8 Montgomery E, Fisher C: Paratesticular liposarcoma: a clinicopathologic study. Am J Surg Pathol 2003;27:40 47. 9 Soosay GN, Parkinson MC, Paradinas J, et al: Paratesticular sarcomas revisited: a review of cases in the British Testicular Tumour Panel and Registry. Br J Urol 1996;77:143 146. 10 Schwartz SL, Swierzewski SJ 3rd, Sondak VK, et al: Liposarcoma of the spermatic cord: report of 6 cases and review of the literature. J Urol 1995;153:154157. 11 Catton CN, Cummings BJ, Fornasier V, et al: Adult paratesticular sarcomas: a review of 21 cases. J Urol 1991;146:342345. 12 Dotan ZA, Tal R, Golijanin D, et al: Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol 2006; 176:20382039. 13 Hermans BP, Foster RS, Bihrle R, et al: Is retroperitoneal lymph node dissection necessary for adult paratesticular rhabdomyosarcoma? J Urol 1998;160:20742077. 14 Emerson RE, Ulbright TM: Morphological approach to tumours of the testis and paratestis. J Clin Pathol 2007;60:866880. 15 Sesterhenn IA, Davis CJ Jr: Pathology of germ cell tumors of the testis. Cancer Control 2004;11:374387. 16 Lopes RI, Leite KR, Lopes RN: Paratesticular leiomyosarcoma treated by enucleation. Int Braz J Urol 2006;32:66 67. 17 Connolly SS, DArcy FT, Bredin HC, et al: Value of frozen section analysis with suspected testicular malignancy. Urology 2006; 67:162165. 18 Enoch S, Wharton SM, Murray DS: Management of leiomyosarcomas of the spermatic cord: the role of reconstructive surgery. World J Surg Oncol 2005;3:2324. 19 Bacci G, Ferrari S, Longhi A, et al: Prognostic significance of serum LDH in Ewings sarcoma of bone. Oncol Rep 1999;6:807811. 20 Blyth B, Mandell J, Bauer SB, et al: Paratesticular rhabdomyosarcoma: results of therapy in 18 cases. J Urol 1990;144:14501453. 21 Catton C, Jewett M, OSullivan B, et al: Paratesticular sarcoma: failure patterns after definitive local therapy. J Urol 1999;161:1844 1847. 22 Khoubehi B, Mishra V, Ali M, et al: Adult paratesticular tumours. BJU Int 2002;90: 707715.
Free Author Copy for personal use only
ANY DISTRIBUTION OF THIS ARTICLE WITHOUT WRITTEN CONSENT FROM S. KARGER AG, BASEL IS A VIOLATION OF THE COPYRIGHT. Written permission to distribute the PDF will be granted against payment of a permission fee, which is based on the number of accesses required. Please contact permission@karger.ch
452
Urol Int 2009;82:448452
Korkes/Castro/Romero/Godoy/Amary/ Fernandes/Perez
Free Author Copy for personal use only
ANY DISTRIBUTION OF THIS ARTICLE WITHOUT WRITTEN CONSENT FROM S. KARGER AG, BASEL IS A VIOLATION OF THE COPYRIGHT. Written permission to distribute the PDF will be granted against payment of a permission fee, which is based on the number of accesses required. Please contact permission@karger.ch
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Bahan AmosDocument3 pagesBahan AmosfaberNo ratings yet
- Major R Cance Er Miles Stones: Overvie EwDocument14 pagesMajor R Cance Er Miles Stones: Overvie Ewtkobosz4877No ratings yet
- Esophageal Cancer: Diagnosis and Management: Ai Zheng Aizheng Chinese Journal of Cancer October 2010Document13 pagesEsophageal Cancer: Diagnosis and Management: Ai Zheng Aizheng Chinese Journal of Cancer October 2010Al VlaovicNo ratings yet
- ICD O MorphologyDocument6 pagesICD O MorphologyJonathan IngramNo ratings yet
- Breast Cancer in Pakistan - Awareness and Early DetectionDocument3 pagesBreast Cancer in Pakistan - Awareness and Early DetectionMurk NiazNo ratings yet
- What is acanthosis nigricans skin conditionDocument2 pagesWhat is acanthosis nigricans skin conditionIulianaNo ratings yet
- Management of The Abnormal Pap SmearDocument29 pagesManagement of The Abnormal Pap SmearMakWohWeiNo ratings yet
- 7.016 Recitation 17 - Fall 2018: Summary of Lectures 25 (11/9) & 26 (11/14)Document9 pages7.016 Recitation 17 - Fall 2018: Summary of Lectures 25 (11/9) & 26 (11/14)Shubham singhNo ratings yet
- Cancer Ovarian - Prezentare de Caz PDFDocument26 pagesCancer Ovarian - Prezentare de Caz PDFHogea OctavNo ratings yet
- Research Paper Niaylah MeansDocument35 pagesResearch Paper Niaylah Meansapi-401164633No ratings yet
- Skin CancerDocument14 pagesSkin Cancerchristian_12No ratings yet
- Prostate Cancer Symptoms, Screening, Stages and Treatment OptionsDocument45 pagesProstate Cancer Symptoms, Screening, Stages and Treatment OptionsNeethiselvam Devadoss100% (2)
- Breast Disorders Assessment GuideDocument26 pagesBreast Disorders Assessment Guideluna nguyenNo ratings yet
- Intestinal Polyps and PolyposisDocument244 pagesIntestinal Polyps and PolyposisVladislav KotovNo ratings yet
- Duodenal Epithelial Polyps: A Clinicopathologic ReviewDocument16 pagesDuodenal Epithelial Polyps: A Clinicopathologic ReviewsilvanaNo ratings yet
- Exploring cancer signs, causes, prevention and treatmentDocument8 pagesExploring cancer signs, causes, prevention and treatmentiyannaNo ratings yet
- Oncology Lectures 1 7 DR - FerrolinoDocument24 pagesOncology Lectures 1 7 DR - FerrolinoMiguel Cuevas DolotNo ratings yet
- Key Stats About Nasopharyngeal CancerDocument2 pagesKey Stats About Nasopharyngeal CancerCharo FloresNo ratings yet
- Haroon RashidDocument50 pagesHaroon RashidSaayk HabibNo ratings yet
- Relationship of urine LRG-1 protein levels with cervical cancer stage and characteristicsDocument17 pagesRelationship of urine LRG-1 protein levels with cervical cancer stage and characteristicsKhumaira SantaNo ratings yet
- Bilateral CA ToungeDocument5 pagesBilateral CA ToungeDrBadrinarayan SharmaNo ratings yet
- ASCO 2017 Edbook PDFDocument871 pagesASCO 2017 Edbook PDFf2ko4100% (2)
- Oncogenic VirusesDocument83 pagesOncogenic VirusespthamainiNo ratings yet
- Tesis FixDocument77 pagesTesis FixRio JuandaNo ratings yet
- Descriptive epidemiology of breast tumor and cancer casesDocument6 pagesDescriptive epidemiology of breast tumor and cancer casesYochanan meisandroNo ratings yet
- Komatsu 2020Document5 pagesKomatsu 2020Felipe RohamNo ratings yet
- Livingston - Neoplastic Africa - Mapping Circuits of Toxicity and KnowledgeDocument23 pagesLivingston - Neoplastic Africa - Mapping Circuits of Toxicity and KnowledgeTanima The-fireflyNo ratings yet
- Everything You Need to Know About Breast CancerDocument27 pagesEverything You Need to Know About Breast Cancerrikshya k c100% (3)
- A Study To Assess The Knowledge Regarding Cervical Cancer Among Women in Selected Community Setting, ChennaiDocument3 pagesA Study To Assess The Knowledge Regarding Cervical Cancer Among Women in Selected Community Setting, ChennaiEditor IJTSRDNo ratings yet