Professional Documents
Culture Documents
Fossile Fuel Draft
Uploaded by
Fatemeh MostofiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fossile Fuel Draft
Uploaded by
Fatemeh MostofiCopyright:
Available Formats
FOSSIL FUEL POWER GENERATION: ENVIRONMENTAL IMPACTS
Mustafa Mustafa, Fatemeh Mostofi, Bachar Al-Jendi,xxxx School of the Built Environment, Heriot-Watt University Dubai Campus Mustafa Mustafa : mm507@hw.ac.uk : registration number : 091608563 Fatemeh Mostofi : fm137@hw.ac.uk : registration number : 091617071 Bachar Al Jendi : ba149@hw.ac.uk : registration number : 091611736
Abstract this paper aims to discuss the environmental impact of fossil fuel burning on the environment, the different pollutants released in the process, as well as mentioning different ways to mitigate the emissions that result from fossil fuel burning.
Keywords: Fossil fuels, Carbon emissions, Non-renewable energy, Green energy
TABLE OF CONTENTS
Abstract ...................................................................................................................................................... 1 1. Introduction .............................................................................................................................................. 2 2. Fossil fuel.................................................................................................................................................... 2 Coal ............................................................................................................................................................... 2 Natural gas ................................................................................................................................................. 2 Oil................................................................................................................................................................... 3 3. Impact on the environment ................................................................................................................ 3 PRIMARY Impacts: ...................................................................................................................................... 3 3.1 Carbon dioxide .................................................................................................................................. 3 3.2 Carbon monoxide (CO) :................................................................................................................ 4 3.3 Sulphur Oxides .................................................................................................................................. 5 3.4 Nitrogen oxides ................................................................................................................................ 5 3.5 particulate matter............................................................................................................................ 5 Secondery Impacts: ..................................................................................................................................... 6 Greenhouse effect ................................................................................................................................... 6 Acid rain ...................................................................................................................................................... 6 Photochemical smog .............................................................................................................................. 6 Green energy.................................................................................................................................................. 6 Page 1 of 6
Case Study : Fujairah Power Plant ........................................................................................................ 6 Conclusions .................................................................................................................................................... 6 references ....................................................................................................................................................... 6
1. INTRODUCTION
2. FOSSIL FUEL
Fossil fuels constitute a significant repository of carbon buried deep underground. Burning them results in the conversion of this carbon to carbon dioxide, which is then released into the atmosphere.
COAL
Using coal-fired plants to generate electricity produces more greenhouse gases for each resulting watt than using oil or natural gas, but coaloften referred to as a dirty fuelis attractive because it is relatively inexpensive. In countries where there are no emission controls (such as China and India)
http://www.worldcoal.org/coal-the-environment/coal-use-the-environment/
NATURAL GAS
Of all the fossil fuels, natural gas is considered the cleanest. Composed primarily of methane, the principal products of its combustion are CO 2 and water vapor. During combustion, very small amounts of sulfur dioxide and nitrogen oxides are released, but virtually no ash or particulate matter is involved. Because CO2 makes up such a high proportion of greenhouse gas emissions, reducing CO2 emissions would be significant in combating the greenhouse effect and global warming. The combustion of natural gas emits almost 30 percent less CO2 than oil and just under 45 percent less CO2 than coal. One issue concerning the use of natural gas is whether the presence of methanethe principal component of natural gas and a very potent
Page 2 of 6
greenhouse gasmakes global warming significantly worse.
OIL
There are also serious environmental effects that result from the burning of oil, such as acid rain. When oil is burned, sulfur dioxides and nitrogen oxides are created in the atmosphere, and, when they combine with moisture, acid rain is created. When acid rain falls back to Earth, it enters rivers, lakes, and streams and can kill plants, fish, and wildlife. Buildings, statues, and other man-made structures can also be damaged as a result of the chemical reaction that occurs when acid hits them. Many historical buildings worldwide are being eroded by acid rain. As oil and gasoline are burned, CO2 is also a by-product that enters the atmosphere, contributing to global warming.
3. IMPACT ON THE ENVIRONMENT PRIMARY IMPACTS: 3.1 CARBON DIOXIDE
Carbon dioxide is a greenhouse gas. Due to human activities such as the combustion of fossil fuels and deforestation, and the increased release of CO2 from the oceans due to the increase in the Earth's temperature, the concentration of atmospheric carbon dioxide has increased by about 35% since the beginning of the age of industrialization. The burning of fossil fuels is considered to be the largest source of carbon dioxide (CO2) emissions, Sadly, their use is steadily increasing. There is about 50 times as much carbon dissolved in the oceans in the form of CO 2 and CO2 hydration products as exists in the atmosphere. The oceans act as an enormous carbon sink, having "absorbed about one-third of all human-generated CO2 emissions to date. Most of the CO2 taken up by the ocean forms carbonic acid. Some is consumed in photosynthesis by organisms in the water, and a small proportion of that sinks and leaves the carbon cycle. There is considerable concern that as a result of increased CO2 in the atmosphere the acidity of seawater will increase and may adversely affect organisms living in the water. In particular, with increasing acidity, the availability of carbonates for forming shells decreases. Page 3 of 6
One of the biggest dilemmas we face today is developing countries, in their race to modernize and industrialize, are building hundreds of coal-fired power plants and increasing CO2 emissions. According to John Christy and Roy Spencer (University of Alabama in Huntsville) the world has been warming up at 0.21OC/decade 1 This might look like a small amount but studies have shown that even a slight change of (60C) in temperature might give catastrophic results: massive die-offs of vegetation a dramatic decrease in food production increased soil erosion increased desertification temperature increases release of methane hydrate from the oceans bottoms gas that is eight times stronger than carbon dioxide)2
(a greenhouse
3.2 CARBON MONOXIDE (CO) :
The product of incomplete combustion of fossil fuels due to insufficient oxygen supply to enable complete oxidation to carbon dioxide (CO2) Carbon monoxide (CO) is a colourless, tasteless, odourless, toxic gas, it inhibits the blood's ability to carry oxygen to vital organs such as the heart and brain. Continuous exposure to carbon monoxide can cause permanent damage to the body and/or death. Table#1 shows the health effects associated with human exposure to carbon monoxide3:
Exposure Hours 0.5 1 2 4 6 8
CO concentration Perceptible 600 200 100 50 25 25 Sickness 1000 600 300 150 120 100 deadly 2000 1600 1000 400 200 150
1http://www.reportingclimatescience.com/news-stories/article/global-warming-rate-is-014c-
per-decade-says-uah-team.html
2 3
Global warming, fossil fuels and pollution, the future of air quality - Julie kerr casper, page 18 Table from http://www.engineeringtoolbox.com/carbon-monoxide-d_893.html
Page 4 of 6
3.3 SULPHUR OXIDES
Sulphur oxides arise during combustion from oxidation of sulphur in sulphur containing fuels, sulphur oxides can have different impacts on the environment, for instance, when sulphur in fossile fuel reacts with oxygen to form sulphur dioxide as shown in the following equation4 : S + O2 = SO2 Sulphur dioxide mainly affects humans, and is not highly dangerous, it has an annoying odour, and it irritates the eyes, however, when its released to the atmosphere it can react with oxygen to form sulphur trioxide 2 SO2 + O2 = 2 SO3 Sulphur trioxide is considered to be more dangerous to humans than sulphur dioxide as it irritates the respiratory tract, a concentration of 1 part per million or 1ppm of sulphur trioxide can cause coughing and choking. Sulphur trioxide can also dissolve in water to form sulphuric acid that is capable of corroding many materials and can also dissolve readily in rain drops and fall on the earth as acid rain which causes serious damages to agriculture, forests, lakes, ponds, rivers, and ground water.
3.4 NITROGEN OXIDES
Nitrogen oxides produced when nitrogen atoms within the molecules of fuel are oxidised during the combustion process to form nitric oxide (NO). When nitric oxide is emitted to the atmosphere, it reacts with oxygen to form nitrogen dioxide that can cause serious damage to humans, such as inflammation of the lungs, and possible death. Nitrogen oxides will react with water and oxygen to form nitric acid, like sulphuric acid, nitric acid is capable of corroding many materials, nitric acid is also a component of acid rain
3.5 PARTICULATE MATTER
Sulphur oxide Sulphur dioxide SO2 Sulphur trioxide SO3
4
Impact on humans Odour, irritation Coughing, choking
Impact on the environment Form SO3 Sulphuric acid
Global warming, fossil fuels and pollution, the future of air quality - Julie kerr casper, page 11
Page 5 of 6
Nitrogen dioxide NO2
Lung inflammation Death
Acid rain Nitric acid Acid rain
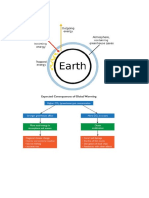
SECONDERY IMPACTS: GREENHOUSE EFFECT
ACID RAIN
PHOTOCHEMICAL SMOG
GREEN ENERGY
http://www.treehugger.com/clean-water/renewable-energy-key-to-makingdesalination-work-for-water-crunched-countries.html
CASE STUDY : FUJAIRAH POWER PLANT
CONCLUSIONS
REFERENCES Sulphur
http://www.ifc.org/ifcext/enviro.nsf/AttachmentsByTitle/p_ppah_SulfurOxides/$FILE /HandbookSulfurOxides.pdf
Page 6 of 6
You might also like
- Document PDFDocument36 pagesDocument PDFIoana Influencers RebornNo ratings yet
- Mba ProjectDocument42 pagesMba ProjectUday GowdaNo ratings yet
- New Microsoft Office Word DocumentDocument14 pagesNew Microsoft Office Word Documentkunkulol100% (1)
- Air PollutionDocument74 pagesAir PollutionSaneet AgrawalNo ratings yet
- CARBON COMPOUNDS: Pollution Aspects: Received Date: Jan. 2020 Revised: April 2020 Accepted: June 2020Document9 pagesCARBON COMPOUNDS: Pollution Aspects: Received Date: Jan. 2020 Revised: April 2020 Accepted: June 2020Vaibhav SiddharthNo ratings yet
- Study of Carbon ScrubberDocument30 pagesStudy of Carbon ScrubberShaeed Bhagat Singh Yuva Club KathuraNo ratings yet
- Folio Biology: Endangered EcosystemDocument23 pagesFolio Biology: Endangered Ecosystemsabrina_shaharNo ratings yet
- Topic:-: Environmental ChemistryDocument18 pagesTopic:-: Environmental ChemistryKaran LachhwaniNo ratings yet
- Adobe Scan Jun 14, 2023Document10 pagesAdobe Scan Jun 14, 2023xoranek474No ratings yet
- Assignment Environmental Economics Carbon Sequestration: Submitted By: Komal Saraogi M.Sc. Agribusiness Economics AE2120Document12 pagesAssignment Environmental Economics Carbon Sequestration: Submitted By: Komal Saraogi M.Sc. Agribusiness Economics AE2120Komal SaraogiNo ratings yet
- Learner'S Packet No. 6 Quarter 1: SDO - HUMSS-TNCTC - Grade 11/12 - Q1 - LP 6Document11 pagesLearner'S Packet No. 6 Quarter 1: SDO - HUMSS-TNCTC - Grade 11/12 - Q1 - LP 6Ralph NavelinoNo ratings yet
- Effects of Carbon Dioxide (CO2) (Final)Document5 pagesEffects of Carbon Dioxide (CO2) (Final)Luis RuizNo ratings yet
- Mitigating Co From Atmosphere Greenearthers Team Members: andDocument13 pagesMitigating Co From Atmosphere Greenearthers Team Members: andAkshat PandeyNo ratings yet
- Climate Change and Its Effect On BangladeshDocument20 pagesClimate Change and Its Effect On Bangladeshsohel479No ratings yet
- Global Warming Potential (GWP)Document5 pagesGlobal Warming Potential (GWP)Ali RazzanNo ratings yet
- Module 2Document11 pagesModule 2Sipra SamuiNo ratings yet
- Personal Skills Development-1: Global WarmingDocument13 pagesPersonal Skills Development-1: Global WarmingFiza MajidNo ratings yet
- Effects of Green House Gases (Chemistry Project Class-11)Document15 pagesEffects of Green House Gases (Chemistry Project Class-11)Anujeet Saha86% (7)
- Module 5Document37 pagesModule 5NANJAPPANo ratings yet
- Global Change Manmade or NaturalDocument5 pagesGlobal Change Manmade or NaturalVamsi KrishnaNo ratings yet
- What Are The Reasons For Global Warming?Document6 pagesWhat Are The Reasons For Global Warming?manirathinaNo ratings yet
- Chemistry EssayDocument2 pagesChemistry Essayapi-410812167No ratings yet
- DADUUUDocument2 pagesDADUUUnehalNo ratings yet
- Green House EffectDocument28 pagesGreen House EffectVyshnavi P VNo ratings yet
- Tugas Bahasa Inggris Tentang Global WarmingDocument10 pagesTugas Bahasa Inggris Tentang Global WarmingRahmatHidayatNo ratings yet
- Mitigation of CO2 by Chemical ConversionDocument21 pagesMitigation of CO2 by Chemical Conversiona_abbaspourNo ratings yet
- Global WarmingDocument13 pagesGlobal Warmingedo adea pratamaNo ratings yet
- Oil and EnvironmentDocument12 pagesOil and EnvironmentLuka ZukicNo ratings yet
- Environmental Pollution: Study MaterialDocument7 pagesEnvironmental Pollution: Study MaterialHemu RaoNo ratings yet
- The Greenhouse EffectDocument3 pagesThe Greenhouse Effectahmed2955No ratings yet
- Anthropogenic Activities and Greenhouse GasesDocument6 pagesAnthropogenic Activities and Greenhouse GasesAWADHI NGELANo ratings yet
- Greenhouse GasesDocument21 pagesGreenhouse GasesSmn619No ratings yet
- Global WarmingDocument4 pagesGlobal WarmingAramae DagamiNo ratings yet
- Green Chemistry ProjectDocument4 pagesGreen Chemistry Projectapi-320290632No ratings yet
- Global WarmingDocument4 pagesGlobal WarmingAbishek KumarNo ratings yet
- 2015 tee-EIS Chapter 3 - Technology Environment PDFDocument81 pages2015 tee-EIS Chapter 3 - Technology Environment PDFYu Gen XinNo ratings yet
- Module 5 - E & EDocument15 pagesModule 5 - E & ECRAZY ಕನ್ನಡಿಗNo ratings yet
- Global WarmingDocument10 pagesGlobal WarmingRahmatHidayatNo ratings yet
- Discuss The Impact That The Human Race Have Had On Planet Earth Since The Industrial Revolution 250 Years AgoDocument5 pagesDiscuss The Impact That The Human Race Have Had On Planet Earth Since The Industrial Revolution 250 Years AgoThe Wooden ChickenNo ratings yet
- Group 2aDocument24 pagesGroup 2aPrince Abu100% (1)
- Investigatory Project of Biology 1 VRSDocument14 pagesInvestigatory Project of Biology 1 VRSAina shivhareNo ratings yet
- BCHE 111L - ULO3aDocument5 pagesBCHE 111L - ULO3aKaris DemetriaNo ratings yet
- Sustainable EngineeringDocument15 pagesSustainable EngineeringAbhijeeth AnilkumarNo ratings yet
- Integrating Act. Chemistry S1Document7 pagesIntegrating Act. Chemistry S1Yisus Alberto Molina ZuñigaNo ratings yet
- Global Warming & Ozone LayerDocument12 pagesGlobal Warming & Ozone LayerWayne JohannesNo ratings yet
- Figure 1: Common Air Pollutants: Environmental Planning & PracticeDocument4 pagesFigure 1: Common Air Pollutants: Environmental Planning & Practiceadeel raziNo ratings yet
- Air PollutionDocument16 pagesAir PollutionVara PrasadNo ratings yet
- Presentation 10Document15 pagesPresentation 10xoranek474No ratings yet
- Methane: Properties and UsesDocument7 pagesMethane: Properties and UsesFahad kamranNo ratings yet
- Presentasi PPD B.inggDocument2 pagesPresentasi PPD B.inggMiLloleNo ratings yet
- Air PollutionDocument33 pagesAir PollutionKapil BudasanaNo ratings yet
- Effects On Bio-Geo-Chemical Cycles: Increased Solar UV Radiation Could Affect Terrestrial andDocument1 pageEffects On Bio-Geo-Chemical Cycles: Increased Solar UV Radiation Could Affect Terrestrial andKashyap DubeyNo ratings yet
- 17 Eng06 001 Energy Tech Mee 543Document7 pages17 Eng06 001 Energy Tech Mee 543Cold LarvaNo ratings yet
- Chemistry AirDocument2 pagesChemistry AirREAL GAMERNo ratings yet
- Global WarmingDocument8 pagesGlobal WarmingsamanNo ratings yet
- Greenhouse Gases: Climate Change Is Real - Causes and Impacts of Climate ChangeDocument15 pagesGreenhouse Gases: Climate Change Is Real - Causes and Impacts of Climate ChangeAhmad Hassan ChathaNo ratings yet
- Environmental Chemistry Class-XiDocument15 pagesEnvironmental Chemistry Class-XiSatyaSaraswatNo ratings yet
- Understanding Global WarmingDocument3 pagesUnderstanding Global WarmingRobbyNo ratings yet
- 36 ch16 PDFDocument6 pages36 ch16 PDFMallikarjuna MuthyaluNo ratings yet
- EST ProjectDocument17 pagesEST Projectanjali bondarNo ratings yet
- Kirk Orthmer UreaDocument19 pagesKirk Orthmer UreaGiovanni JonathanNo ratings yet
- Els - q2 - Quiz 2Document2 pagesEls - q2 - Quiz 2Zaira Kimberly SantiagoNo ratings yet
- Chemistry Powerpoint (Autosaved)Document57 pagesChemistry Powerpoint (Autosaved)Zeedan MohammedNo ratings yet
- Chemical Process Safety: Chapter 6: Fires & ExplosionsDocument41 pagesChemical Process Safety: Chapter 6: Fires & Explosionsiamayesha725No ratings yet
- MSDS H2 PDFDocument10 pagesMSDS H2 PDFrenardiandhika2137No ratings yet
- Dwnload Full Contemporary Logistics 12th Edition Murphy Solutions Manual PDFDocument35 pagesDwnload Full Contemporary Logistics 12th Edition Murphy Solutions Manual PDFabsence.bruitb4vy100% (14)
- Nitrogen Gas SystemDocument4 pagesNitrogen Gas Systemmilton1987No ratings yet
- Spectrometric Determination of NO in Ambient Air and Its ControlDocument6 pagesSpectrometric Determination of NO in Ambient Air and Its ControlMutiaKhairunnisaNo ratings yet
- 545 ChemistryDocument24 pages545 Chemistrykitderoger_391648570No ratings yet
- Cortec India NACE - SZ and CII Sept, 2014Document70 pagesCortec India NACE - SZ and CII Sept, 2014harishkumar.ravichandranNo ratings yet
- A Diagnostic Study in A Husk Fired Boiler For A Power PlantDocument44 pagesA Diagnostic Study in A Husk Fired Boiler For A Power Plantparthi20065768No ratings yet
- Smart Sensor 4 Gases AS8900 - Manual de OperaçãoDocument12 pagesSmart Sensor 4 Gases AS8900 - Manual de OperaçãoRogerioPascualNo ratings yet
- 40 Inventive Principles With ExamplesDocument7 pages40 Inventive Principles With ExamplesJM CuevasNo ratings yet
- The Bunsen Burner: Air Hole OpenDocument1 pageThe Bunsen Burner: Air Hole OpenLeena BhaiNo ratings yet
- Liquefied Petroleum Gas (LPG) : 1. Identification of The Material and SupplierDocument6 pagesLiquefied Petroleum Gas (LPG) : 1. Identification of The Material and SupplierstefenandreanNo ratings yet
- 1 - Atoms, Molecules and StoichiometryDocument80 pages1 - Atoms, Molecules and StoichiometryHenry ChongNo ratings yet
- First Steps Toward SpaceDocument320 pagesFirst Steps Toward SpaceCliff ThriveNo ratings yet
- Boiler For CLC-mainDocument13 pagesBoiler For CLC-mainAnonymous 2g4jKo5a7vNo ratings yet
- NIT KukDocument908 pagesNIT Kukrajmeet singhNo ratings yet
- Oxidation Methods of Activated CarbonDocument8 pagesOxidation Methods of Activated CarbonclaudjiuNo ratings yet
- ?????Document89 pages?????munglepreeti2No ratings yet
- Acid Dewpoint - Acidic Dewpoint CorrelationDocument10 pagesAcid Dewpoint - Acidic Dewpoint CorrelationGary Jones100% (1)
- 2018 Sec 4 Pure Chem Exam Paper PDFDocument487 pages2018 Sec 4 Pure Chem Exam Paper PDFMaverickNo ratings yet
- Sample of Research ReportDocument6 pagesSample of Research Reportapi-314423329No ratings yet
- PS AFF 5 FireBehaviorPowerPointDocument60 pagesPS AFF 5 FireBehaviorPowerPointBarbosaNascimentoNo ratings yet
- NitroxDocument19 pagesNitroxŞef Utas AwijNo ratings yet
- Evolution and BiodiversityDocument4 pagesEvolution and BiodiversityTrần ĐăngNo ratings yet
- Process Notes: Final ProjectDocument8 pagesProcess Notes: Final ProjectCluisantony Jayco DizeNo ratings yet
- JURNAL 4 CEDERA KEPALA FixDocument8 pagesJURNAL 4 CEDERA KEPALA FixFebrianti FebriantiNo ratings yet