Professional Documents
Culture Documents
Pulse Rate Variability Is Not A Surrogate For Heart Rate Variability
Uploaded by
Firas ZakOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pulse Rate Variability Is Not A Surrogate For Heart Rate Variability
Uploaded by
Firas ZakCopyright:
Available Formats
Clinical Science (1999) 97, 391397 (Printed in Great Britain)
391
Pulse rate variability is not a surrogate for heart rate variability
Isabelle CONSTANT*, Dominique LAUDE, Isabelle MURAT* and Jean-Luc ELGHOZI
*Service dAnesthe! sie Re! animation Pe! diatrique, Ho# pital denfants Armand Trousseau, 26 av. du Dr Arnold Netter, 75571 Paris cedex 12, France, and Centre de Pharmacologie Clinique, Association Claude Bernard, CNRS URA 1482, Ho# pital Necker-Enfants Malades, Paris, France
To investigate the dierences between heart rate (HR) variability and pulse rate (PR) variability, short-term variability of nger pulse wave and ECG signals were studied in 10 children with a xed ventricular pacemaker rhythm (80 beats/min). Ten healthy children in sinus rhythm served as a reference population. Distal PR and HR were measured continuously using a Finapres device and an ECG respectively. Power spectra for HR and PR were calculated in both the supine and orthostatic positions. In paced subjects, PR spectra exhibited the characteristic respiratory peak, although the HR spectra were at. Similarly, in healthy children the respiratory uctuations were more pronounced when calculated from the nger pulse wave signal compared with the ECG signal. The overestimation of HR respiratory uctuation resulting from distal PR measurement was more pronounced in the standing position ; however, this postural eect was demonstrated only in healthy subjects. We observed mechanical respiratory modulation of distal PR independent of classical HR modulations. Our results suggest a mechanical respiratory inuence via cardiac output and aortic transmural pressure changes on pulse wave velocity. We conclude that respiratory PR variability does not precisely reect respiratory HR variability in standing healthy subjects and in patients with low HR variability. Consequently, HR modulation should be studied using the ECG signal rather than the distal pulse wave signal. However, when ECG recording is not available, the distal pulse wave is an acceptable alternative.
INTRODUCTION
Heart rate (HR) is usually calculated from the time elapsed between two ventricular contractions ; in other words, the time between two consecutive R-waves on the ECG (RR interval). This time period is strongly regulated and depends mainly on neural factors such as autonomic control on the sinus node, on hormonal factors such as the reninangiotensin peptides, and on mechanical factors such as right auricular wall stretch. Given that HR is a determinant of stroke volume and
cardiac output, it plays a major role in the regulation of arterial blood pressure. Continuous recording of HR shows regular uctuations that reect parasympathetic and sympathetic neural control on the sinus node. Spectral analysis of the short-term variability of HR allows quantitative assessment of these neurogenic oscillations, and therefore provides indices of neural regulation of HR [1,2]. This non-invasive methodology has numerous clinical applications, specically in physiology, in cardiology and in pharmacology.
Key words : autonomic nervous system, blood pressure, children, heart rate, pacemaker, pulse rate, respiration, variability, velocity. Abbreviations : HF, high-frequency ; HR, heart rate ; LF, low-frequency ; PR, pulse rate ; PW, pulse wave ; R, beat-to-beat difference between HR and PR. Correspondence : Dr Isabelle Constant (e-mail isabelle.constant!trs.ap-hop-paris.fr).
# 1999 The Biochemical Society and the Medical Research Society
392
I. Constant and others
In clinical practice, HR is routinely obtained from the distal pulse. Indeed, HR is commonly approximated from the pulse rate (PR), estimated from the time between two systolic peaks of pulse wave (PW), i.e. at a radial or digital level. This use of PR as a surrogate for HR requires identity of HR and PR uctuations. Indeed, HR is closely correlated with PR, but small periodic deviations around an accurate mean cannot be excluded. HRindependent PR uctuations are poorly documented. The ideal way to investigate the specic modulation of PR is to suppress HR modulation. In humans, the lack of an inuence of HR on PR can be investigated in a variety of ways : after pharmacological blockade (although drugs may modify the vascular physiological responses) ; after cardiac transplantation (although a small cardiac intrinsic variability may still persist) ; and in patients with xed pacemaker rhythm. Unfortunately, most patients in this nal category have cardiovascular diseases and are undergoing treatment which may interact with the physiological process. To avoid this bias, we studied young subjects with normal cardiac performance, in whom a pacemaker was implanted because of congenital impairment of atrioventricular conduction. To test the hypothesis that PR uctuates independently of HR modulation, we compared HR uctuations with PR uctuations in these paced subjects with xed ventricular rhythm. In addition, we studied age-matched healthy children, in order to evaluate in healthy subjects the relative contribution of this HR-independent modulation of PR. HR was derived simultaneously from the R-wave of the ECG, and from the systolic peak of the digital blood pressure curve (Finapres). These parameters were subjected to spectral analysis as described previously [3]. The study was performed in both the supine and orthostatic positions in order to examine the effects of postural variations on these parameters. Previously we evaluated the consequences of withdrawal of HR uctuations on the short-term regulation of blood pressure in a group of young children with xed ventricular pacemaker rhythm [4]. The study reported herein represents our continued investigations into the consequences of withdrawal of HR uctuations on shortterm blood pressure regulation.
normal cardiac anatomy and left ventricular function, as assessed by echocardiography. None of the patients was taking medication of any type. At 1 h before recordings, the paced children (PM group) were programmed to the VVI mode (xed ventricular rate) at a pacing rate of 80 beats\min.
Study protocol
Children were studied in the morning in a quiet room at 22 mC. Electrocardiograph measurements were taken with disposable electrodes attached to the thorax, placed to provide clear R-waves, and connected to a Datex cardiocap II monitor (Instrumentation Corp, Helsinski, Finland). Finger arterial pressure was monitored noninvasively by a Finapres device (model 2300 ; Ohmeda, Trappes, France). In this study, all children had a nger circumference of between 42 and 60 mm. To ensure optimal Finapres blood pressure measurement, we used appropriate cuff sizes (S or M) according to the manufacturers instructions. The cuff was tted to the third nger of the right hand, which was passively maintained at heart level during the manoeuvres. Two recordings of 5 min duration were obtained ; the rst recording was obtained during 5 min in the supine position after a 10min rest, and the second was taken when the subjects were in orthostatic posture. Breathing was quantied by respiratory inductance plethysmography with a Respitrace device (Ardley, New York, NY, U.S.A.). During recordings obtained in the supine and standing positions, children breathed spontaneously during the rst 1 min and were then asked to control their breathing frequency using an auditory signal from an electronic metronome. The controlled respiratory rate was determined from the spontaneous breathing rate of each child during the rst 1 min of recording. HR variability studies were performed during controlled breathing. The investigation conformed to the principles outlined in the Declaration of Helsinki (1989) of the World Medical Association, and was approved by the local Ethical Committee. The nature of the research was explained to the childrens parents, and informed consent was obtained.
Data processing and spectral analysis
METHODS
Study population
Results from 10 children with an implanted bipolar DDD pacemaker [age 8.7p2.2 years (meanpS.D.), range 613 years ; weight 27.6p10.8 kg] and 10 healthy children with sinus rhythm (age 8.6p2.5 years, range 613.5 years ; weight 28.8p6.0 kg) form the basis of this study. The indication for pacemaker implantation was isolated congenital complete heart block. All paced children had
# 1999 The Biochemical Society and the Medical Research Society
The details of data sampling and analysis have been described previously [2]. The analogue output of the Datex monitor and of the Finapres device was connected to an analogue-to-digital converter to permit data acquisition, storage and analysis using a microcomputer. The blood pressure and ECG signals were digitized (500 Hz) and processed by an algorithm based on feature extraction to detect and measure the characteristics of a blood pressure cycle and an R-wave (Anapres 3.0 ; Notocord Systems, Croissy\Seine, France). According to the sample rate (500 Hz), the precision of signal
Pulse rate variability
393
detection was 0.002 s, which represents the limits of resolution of the methodology. HR was estimated simultaneously from both nger blood pressure and ECG signals, as 60 000 divided by the pulse or heart period in ms, measured from the corresponding systolic peak and the preceding one, and from the corresponding R-wave and the preceding one, respectively. A resampling rate of 10 Hz was chosen without interpolation, i.e. HR values were replicated every 0.1 s until a new blood pressure cycle or R-wave occurred within a 0.1 s window. The evenly spaced sampling allowed direct spectral analysis using a fast Fourier transform (FFT) algorithm on a 2048-point stationary time series. This corresponded to a period of 3 min 25 s. Thus each spectral band corresponded to a harmonic of 5\1024 Hz, or 0.00488 Hz. The power of the HR shown in the Figures had units of (beats\min)#. Modulus (square root of the power) was used for calculations and statistical analysis. The integration of the values of consecutive bands was computed in order to estimate the various components of the variability. As breathing frequency was controlled, the high-frequency (HF) oscillation was easily detected within the 0.20.5 Hz range, and we summed the value of the band corresponding to the respiration and the four values of higher and lower frequency (i.e. nine values) to integrate the HF component of HR. The low-frequency (LF) component was obtained by integration of the values of the consecutive bands from 0.0635 to 0.127 Hz of the HR spectrum, in order to include the 10-s rhythm (0.1 Hz) [5]. We also calculated descriptive statistics (means, S.D.) of the distribution of the HR of each stationary period of 205 s used for spectral analysis. In addition, to analyse further the difference between HR and PR variability, the beat-to-beat difference between HR and PR was re-calculated. Spectral analysis of this parameter was performed after equidistant resampling (10 Hz).
Table 1 Average systolic blood pressure (SBP) values in the supine and standing positions, obtained in healthy controls (group C) and in paced children (group PM)
Included is the S.D. of SBP, and the LF and HF components of the SBP spectra in absolute values (mmHg). Data are meanspS.E.M. Statistical significance of differences between standing and supine : *P 0.05, **P 0.001. Pressure (mmHg) SBP Supine Group PM Group C Standing Group PM Group C 84.3p3.2 76.2p4.5 S.D. of SBP 4.6p0.4 4.5p0.4 SBP LF 1.5p0.2 1.6p0.1 1.7p0.2 2.1p0.2* SBP HF 1.3p0.2 1.1p0.1 1.3p0.2 1.8p0.2**
94.8p2.9 4.6p0.4 99.8p6.2** 5.7p0.6*
the paced subjects and in the healthy children in both postures (Table 1).
Comparisons between HR variability and PR variability Paced subjects
As expected, HR variability was greatly reduced and the corresponding spectra were dramatically at in paced subjects ; accordingly, PR variability was markedly reduced. However, PR exhibited small uctuations with respiration ; indeed, the corresponding spectra showed a respiratory peak signicantly more pronounced than in the HR spectra (P 0.001) (Table 2).
Control subjects
Healthy children exhibited a high degree of HR variability and PR variability, as estimated by the S.D. of the distribution ranging between 4 and 10 beats\min (Table 2). Frequency domain analysis of these variabilities revealed classical components, mainly assessed by the LF and the respiratory (HF) peak of the power spectra. The respiratory peak (HF) of PR variability was more pronounced compared with the respiratory peak of HR variability (P 0.001), while the LF components of HR variability and PR variability were similar.
Statistical analysis
Data are expressed as meanspS.E.M. Signicant differences between PR and HR, and between postures, were determined by one-way analysis of variance for repeated measures. When the F-value of the analysis of variance was signicant, differences between groups and postures were tested using the modied t-statistics based on the Bonferroni method. Differences were considered to be statistically signicant when the P value was 0.05.
Variability of the beat-to-beat difference between HR and PR (R )
In all subjects, R displayed respiratory uctuations (Table 3). Spectral analysis of beat-to-beat calculation of R led to characteristic spectra with a single peak located at the respiratory frequency (HF) (Figure 1). In the control group, overall variability (S.D.) and the HF peak were increased in the standing position (respectively P l 0.001 and P l 0.024), while in the PM group this postural effect was not signicant.
# 1999 The Biochemical Society and the Medical Research Society
RESULTS
Blood pressure data
Average levels of systolic blood pressure and spectral proles of systolic blood pressure were comparable in
394
I. Constant and others
Table 2 Average HR and PR values in the supine and standing positions, obtained in healthy controls (group C) and in paced children (group PM)
Included are the S.D.s of HR and PR, and the LF and HF components of the HR and PR spectra in absolute values (beats/min). Data are meanspS.E.M. Statistical significance of differences between HR and PR : **P 0.001. Rate (beats/min) HR Group C Supine Standing Group PM Supine Standing 84.5p4.2 102.9p5.6 80.2p0.0 80.3p0.1 S.D. of HR 7.4p0.5 6.4p0.4 0.1p0.0 0.3p0.1 PR 84.4p4.2 102.9p5.6 80.3p0.1 80.3p0.1 S.D. of PR 7.5p0.5 6.7p0.4 0.8p0.1 0.8p0.1 HR LF 2.6p0.3 2.5p0.3 0.0p0.0 0.0p0.0 PR LF 2.7p0.3 2.5p0.3 0.1p0.0 0.1p0.0 HR HF 2.8p0.4 2.0p0.4 0.1p0.0 0.1p0.0 PR HF 3.1p0.4** 2.5p0.4** 0.4p0.1** 0.4p0.0**
Table 3 Average R values in the supine and standing positions, calculated in healthy controls (group C) and in paced children (group PM)
Included is the S.D. of R, and the LF and HF components of the R spectra in absolute values (beats/min). Data are meanspS.E.M. Statistical significance of differences between standing and supine : *P 0.05, **P 0.001. Difference (beats/min) R Group C Supine Standing Group PM Supine Standing 0.05p0.01 0.04p0.01 0.02p0.00 0.02p0.00 S.D. of R R LF R HF 0.41p0.04 0.60p0.07* 0.44p0.05 0.35p0.03
0.82p0.38 0.09p0.01 1.19p0.47** 0.09p0.01 0.81p0.30 0.80p0.34 0.07p0.00 0.06p0.01
DISCUSSION
Young subjects implanted with a cardiac pacemaker for the alleviation of a congenital impairment of atrioventricular conduction, but with no other clinical indications, provide a clinical model whereby the uctuations of PR can be assessed independently of those of HR. In the present study we provide evidence documenting respiratory uctuations of PR independent of uctuations of HR, and an increase in the magnitude of these uctuations in the standing position in healthy subjects only. Therefore assessment of HR variability from PR may lead to an overestimation of the HF component of HR variability, by as much as 25 % in standing healthy subjects and by 300 % in subjects with xed ventricular rhythm. The present study supports the results published by Karrakchou et al. [6] suggesting that the spectra of HR calculated from the ECG and nger blood pressure signals differed for high frequencies. The respiratory uctuations of PR independent of uctuations of HR were demonstrated using the nger
# 1999 The Biochemical Society and the Medical Research Society
PW signal. Our results might be specic to measurement of PR at a distal site. The detection of the systolic peak from the blood pressure signal was processed by a complex algorithm including a sequence of mathematical validations based on derivative processes. A minimal respiratory-induced change in the shape of the blood pressure waveform peak cannot be excluded as making a possible contribution to the differences observed. In paced subjects programmed in the VVI mode, the cardiac electrical impulse is regularly generated at the level of the right ventricle, and if we assume that variations of electromechanical coupling are negligible, HR does not uctuate. Hence the time interval between two consecutive systolic ejections may be considered as constant. Under these conditions, the existence of periodic uctuations of distal PR must be due to periodic uctuations in the time taken for the arterial pulse pressure wave (shock wave) to be generated from the electrical impulse and to travel from the aortic valve to a peripheral site. While the pre-ejection time (left ventricular isometric contraction) depends mainly on left ventricular contractility, the duration of propagation of the PW depends on PW velocity [7]. Hence periodic variations in distal PR might result from periodic changes in isometric contraction time or in PW velocity, or both. In healthy adults the pre-ejection time is usually of the order of 50 ms, while the propagation time of the PW is approx. 250 ms [8]. Respiratory-induced changes in ventricular loading conditions may result in respiratorydependent variability of the pre-ejection time via modulation of the mechanoelectric feedback [9]. However, the difculty in detecting the exact moment of opening of the aortic valve non-invasively, along with the relatively small contribution of the pre-ejection time, led us to only discuss the involvement of periodic changes in the PW propagation time in the genesis of distal PR uctuations. We hypothesized that periodic uctuations in distal PR result mainly from periodic uctuations of PW velocity. Given that stiffness and tension in the arterial wall are the main factors determining the speed of
# 1999 The Biochemical Society and the Medical Research Society
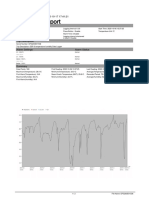
Examples of HR, PR, R and respiration recordings in one 10-year-old normal child (left) and in one 10-year-old child with a fixed ventricular rhythm (right) Recordings were obtained in the standing position, and the corresponding power spectra are shown. Note that the scales for the spectral analysis of HR and PR differ by a factor of 500 between the healthy child and the paced child. Figure 1
Pulse rate variability 395
396
I. Constant and others
transmission of the PW, several mechanisms may be suggested to explain our results. Periodic uctuations of PW velocity may be related to active changes in arterial compliance resulting from a central modulation, such as sympathetic baroreex-mediated oscillations, or from local modulation, such as spontaneous oscillations of small arteries. It may also be hypothesized that periodic uctuations of PW velocity result from passive changes in cardiovascular loading conditions via a mechanical inuence of respiration, such as variations in intrathoracic vessel transparietal pressure and in systolic ejection volume. PW velocity varies inversely with the arterial compliance. As such, any increase in vessel stiffness (decreased compliance) increases PW velocity. Changes in PW velocity might be the consequence of sympathetic vasomotor uctuations. Direct measurements of efferent muscle sympathetic nerve activity have demonstrated that the sympathetic nervous activity consists of rhythmic oscillations [10]. In human, both LF (around 0.1 Hz) and HF (respiratory frequency) components have been detected [11]. The oscillation characteristics of muscle sympathetic nerve activity were closely mirrored by oscillations of systolic blood pressure [12]. Respiratory uctuations in PR might be due to sympathetic-mediated and respiratory-induced changes in vascular compliance ; however, we failed to demonstrate any LF uctuation of PR in our study. The respiratory specicity of PR uctuations seems to rule out the involvement of sympathetic-mediated alterations in vascular compliance. The site of measurement might be taken into consideration as a potential factor involved in the genesis of the unexplained periodicity of the PR. Indeed, the PW signal was recorded using a digital photoplethysmographic device. The digital arteries under the cuff may be subject to rhythmic contractions. Several rhythms have been demonstrated in smooth muscle tone in human arteries [13,14]. Cyclic vasomotor oscillations observed at frequencies ranging between 0.02 and 0.15 Hz may act independently as local spontaneous oscillations, or synchronously as a driving sympathetic rhythm. However, these peripheral vasomotor rhythms seem to predominate at lower frequencies ( 0.15 Hz) [15], and consequently are probably not involved in the mechanism accountable for the PR respiratory variability. Respiration strongly inuences arterial pressure. Respiration-induced changes in blood pressure result from changes in cardiac output, probably via respirationinduced changes in HR and ventricular lling [16,17]. Jurgen and co-workers [1820] have extensively studied the normal decreases in both arterial pressure and left ventricular stroke volume that occur with an inspiratory decrease in intrathoracic pressure. They concluded that the phenomenon is due to independent effects of negative intrathoracic pressure on both left ventricular preload and afterload [18]. They suggested that the decreases in
# 1999 The Biochemical Society and the Medical Research Society
peripheral arterial ow and pressure (pulsus paradoxus) with negative intrathoracic pressure are associated with a diminished left ventricular output, together with retained blood within the intra-arterial compartment [19]. They also demonstrated that the negative intrathoracic pressure led to an increase in aortic cross-sectional area, a decrease in thoracic aortic pressure and a decrease in anterograde ow (or even retrograde ow) in the descending aorta [20]. These ndings, which Olsen et al. referred to as a reverse thoracic pump [21] may support a mechanism for a decrease in PW velocity on inspiration. With differences between HR and PR apparently strongly and specically related to respiratory uctuations in systolic blood pressure, we hypothesize that changes in intrathoracic pressure, and subsequent uctuations in cardiac output and aortic loading conditions, are involved in the genesis of the differences between HR variability and PR variability. In agreement with our ndings, Pitson and co-workers [22,23] demonstrated that the inspiratory rise in pulse transit time (measured from the R-wave to the nger PW) correlates well with the degree of inspiratory effort. The mechanical coupling between respiratory activity and vasculature within the thorax is stronger in the standing than in the supine position [17]. In agreement with this nding, we demonstrated an orthostatic accentuation of the difference between respiratory HR variability and respiratory PR variability. However, this postural effect was evident only in healthy children. We have found previously that, in paced children, the lack of autonomic control on the sinus node was associated with a reduction in the magnitude of the changes in systolic blood pressure variability induced by the standing position [4]. We hypothesized that the orthostatic accentuation of systolic blood pressure in control subjects might be due to marked variations in HR, resulting in cardiac output and subsequent blood pressure variations, and we concluded that, in normal children, HR uctuations increase the systolic blood pressure variability rather than buffering it. These ndings are consistent with the involvement of variations in HR, via cardiac output changes, in the magnitude of the difference between HR and PR respiratory variability. However, as HR itself is a major determinant of PW velocity, we cannot eliminate a potential inuence of the posturerelated increase in average HR levels on the orthostatic accentuation of the difference between respiratory HR variability and respiratory PR variability. In conclusion, we have observed a mechanical respiratory modulation of PR, which acts independently of classical HR modulations. Our results might testify to a mechanical respiratory inuence, via cardiac output and aortic transmural pressure changes, on PW velocity. Consequently, PR variability does not exactly reect HR variability, especially in standing healthy subjects and in patients with low HR variability. However, under several
Pulse rate variability
397
conditions, PR may represent an acceptable surrogate signal for HR, provided that care is taken to avoid subjects that show either very high or very low HR variability.
REFERENCES
1 Akselrod, S., Gordon, D., Madwed, J. B., Snidman, N. C., Shannon, D. C. and Cohen, R. J. (1985) Hemodynamic regulation : investigation by spectral analysis. Am. J. Physiol. 249, H867H875 2 Pomeranz, B., Macaulay, R. J., Caudill, M. A. et al. (1985) Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. 248, H151H153 3 Constant, I., Girard, A., Le Bidois, J., Villain, E., Laude, D. and Elghozi, J. L. (1995) Spectral analysis of systolic blood pressure and heart rate after heart transplantation in children. Clin. Sci. 88, 95102 4 Constant, I., Villain, E., Laude, D., Girard, A., Murat, I. and Elghozi, J. L. (1998) Heart rate control of blood pressure variability in children : a study in subjects with xed ventricular rhythm. Clin. Sci. 95, 3342 5 Jaffe, R. S., Fung, D. L. and Behrman, K. H. (1994) Optimal frequency ranges for extracting information on autonomic activity from the heart rate spectrogram. J. Auton. Nerv. Syst. 46, 3746 6 Karrakchou, M., Vesin, J. M., Laberer, S. and Pruvot, E. (1992) Analysis of heart rate variability : comparison between spectra obtained from ECG and nger blood pressure. Proc. Annu. Int. Conf. IEE Eng. Med. Biol. Soc. 14, 559560 7 Gosse, P., Guillo, P., Ascher, G. and Clementy, J. (1994) Assessment of arterial distensibility by monitoring the timing of Korotkoff sounds. Am. J. Hypertens. 7, 228233 8 Katz, A. M. (1986) Cardiac muscle : structure and function. In Physiology of the Heart, pp. 124, Raven Press, New York 9 Taggart, P. (1996) Mechano-electric feedback in the human heart. Cardiovasc. Res. 32, 3843 10 Eckberg, D. L., Nerhed, C. and Wallin, B. G. (1985) Respiratory modulation of muscle sympathetic and vagal cardiac outow in man. J. Physiol. (London) 365, 181196
11 Saul, J. P., Rea, R. F., Eckberg, D. L., Berger, R. D. and Cohen, R. J. (1990) Heart rate and muscle sympathetic nerve variability during reex changes of autonomic activity. Am. J. Physiol. 258, H713H721 12 Pagani, M., Lombardi, F., Guzzetti, S. et al. (1986) Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 59, 178193 13 Hayoz, D., Tardy, Y., Rutschmann, B. et al. (1993) Spontaneous diameter oscillations of the radial artery in humans. Am. J. Physiol. 264, H2080H2084 14 Rizzoni, D., Castellano, M., Porteri, E. et al. (1995) Arterial spontaneous rhythmic contractile activity in humans and rats : spectral analysis and regulatory mechanisms. J. Hypertens. 13, 10431052 15 Bernardi, L., Hayoz, D., Wenzel, R. et al. (1997) Synchronous and baroceptor-sensitive oscillations in skin microcirculation : evidence for central autonomic control. Am. J. Physiol. 273, H1867H1878 16 Saul, J. P., Berger, R. D., Chen, M. H. and Cohen, R. J. (1989) Transfer function analysis of autonomic regulation. II. Respiratory sinus arrhythmia. Am. J. Physiol. 256, H153H161 17 Saul, J. P., Berger, R. D., Albrecht, P., Stein, S. P., Chen, M. H. and Cohen, R. J. (1991) Transfer function analysis of the circulation : unique insights into cardiovascular regulation. Am. J. Physiol. 261, H1231H1245 18 Jurgen, P., Fraser, C., Stuart, S., Baumgartner, W. and Robotham, J. L. (1989) Negative intrathoracic pressure decreases independently left ventricular lling and emptying. Am. J. Physiol. 257, H120H131 19 Jurgen, P., Kindred, M. K. and Robotham, J. L. (1988) Transient analysis of cardiopulmonary interactions. II. Systolic events. J. Appl. Physiol. 64, 15181526 20 Jurgen, P., Kindred, M. K. and Robotham, J. L. (1988) Transient analysis of cardiopulmonary interactions. I. Diastolic events. J. Appl. Physiol. 64, 15061517 21 Olsen, C. O., Tyson, G. S., Maier, G. W., Davis, J. W. and Rankin, F. S. (1985) Diminished stroke volume during inspiration : a reverse thoracic pump. Circulation 72, 668679 22 Pitson, D. J., Sandell, A., Van den Hout, R. and Stradling, J. R. (1995) Use of pulse transit time as a measure of inspiratory effort in patients with obstructive sleep apnoea. Eur. Respir. J. 8, 16691674 23 Pitson, D. J. and Stradling, J. R. (1998) Value of beat-tobeat blood pressure changes, detected by pulse transit time, in the management of the obstructive sleep apnoea\hypopnoea syndrome. Eur. Respir. J. 12, 685692
Received 15 February 1999/13 May 1999; accepted 11 June 1999
# 1999 The Biochemical Society and the Medical Research Society
You might also like
- Hustler Mini Z 44/52 Parts ManualDocument125 pagesHustler Mini Z 44/52 Parts ManualGary0% (1)
- Heart Rate VariabilityDocument22 pagesHeart Rate VariabilityJyoti Kathwal100% (1)
- BioRadio - WirelessECG PDFDocument13 pagesBioRadio - WirelessECG PDFsastrakusumawijayaNo ratings yet
- Ajr 11 6559Document4 pagesAjr 11 6559g1381821No ratings yet
- 5157024Document11 pages5157024pantufoNo ratings yet
- The Relationship Between Resting Heart Rate Variability and Heart Rate RecoveryDocument6 pagesThe Relationship Between Resting Heart Rate Variability and Heart Rate RecoveryAndrés MenesesNo ratings yet
- Relation Between Respiratory Rate and HeDocument4 pagesRelation Between Respiratory Rate and HeMuhammad Naufal NoorrachmanNo ratings yet
- Heart Rate Variability: A Diagnostic and Prognostic Tool in Anesthesia and Intensive CareDocument15 pagesHeart Rate Variability: A Diagnostic and Prognostic Tool in Anesthesia and Intensive CarenrlaprilianiNo ratings yet
- Pilot Study of The Effectiveness of Butterfly Technique of SCENAR Therapy On Heart Rate VariabilityDocument7 pagesPilot Study of The Effectiveness of Butterfly Technique of SCENAR Therapy On Heart Rate VariabilityParallaxsterNo ratings yet
- Assessment of Palpitation Complaints Benign Paroxysmal Positional VertigoDocument6 pagesAssessment of Palpitation Complaints Benign Paroxysmal Positional VertigoHappy PramandaNo ratings yet
- Jurnal YaahDocument9 pagesJurnal YaahTatiex 'logat' NoerhayatiNo ratings yet
- 1996 Nonlinear Control of Heart Rate Variability in Human InfantsDocument6 pages1996 Nonlinear Control of Heart Rate Variability in Human InfantsSP507No ratings yet
- Linear and Non-Linear Analyses of Heart Rate Variability: A MinireviewDocument9 pagesLinear and Non-Linear Analyses of Heart Rate Variability: A MinireviewMarkoCar16No ratings yet
- L Malmqvist (ESPAÑOL PUMED)Document5 pagesL Malmqvist (ESPAÑOL PUMED)adr3an.tabaresNo ratings yet
- Epilesia 1Document1 pageEpilesia 1Lucilia PereiraNo ratings yet
- Impact of Reduced Heart Rate Variability On Risk For Cardiac EventsDocument14 pagesImpact of Reduced Heart Rate Variability On Risk For Cardiac EventsRakibul HasanNo ratings yet
- 08 Chapter 2Document10 pages08 Chapter 2surbhi pareekNo ratings yet
- Time and Frequency Domain Analysis of Heart Rate VariabilityDocument7 pagesTime and Frequency Domain Analysis of Heart Rate VariabilityArchana TatavartiNo ratings yet
- HRV UtkarshaDocument68 pagesHRV UtkarshaVinita SinghNo ratings yet
- Monitoring of Nocturnal Central Sleep Apnea in Heart FailureDocument5 pagesMonitoring of Nocturnal Central Sleep Apnea in Heart FailureArda Safira Kardono D4AJNo ratings yet
- Abbott2005 HospitalaresDocument8 pagesAbbott2005 HospitalaresMary Marlene Tarazona MolinaNo ratings yet
- Cardiac Sympathovagal Balance During Sleep Apnea Episodes: Vanninen, A. Tuunainen, M. Kansanen,? Uusitupas and LansimiesDocument8 pagesCardiac Sympathovagal Balance During Sleep Apnea Episodes: Vanninen, A. Tuunainen, M. Kansanen,? Uusitupas and LansimiesaudiNo ratings yet
- Breath Holding Index in The Evaluation of Cerebral VasoreactivityDocument5 pagesBreath Holding Index in The Evaluation of Cerebral Vasoreactivityydhapratama11No ratings yet
- Chronobiology of Rate Pressure Product in Young AdultsDocument4 pagesChronobiology of Rate Pressure Product in Young AdultsIjsrnet EditorialNo ratings yet
- Bezold JarischDocument6 pagesBezold Jarischbecky_meloniNo ratings yet
- Heart Rate Variability DissertationDocument8 pagesHeart Rate Variability DissertationWriteMyPaperFastSingapore100% (1)
- Chronic Inappropriate Sinus Tachycardia (Lopera)Document5 pagesChronic Inappropriate Sinus Tachycardia (Lopera)Raymond BernardusNo ratings yet
- ResuscitationDocument7 pagesResuscitationKelvin AKNo ratings yet
- Hipotermi 9Document4 pagesHipotermi 9wahyuartyningsihNo ratings yet
- The Effects of Age On Neural Blockade and Hemodynamic Changes After Epidural Anesthesia With RopivacaineDocument6 pagesThe Effects of Age On Neural Blockade and Hemodynamic Changes After Epidural Anesthesia With RopivacaineAnonymous K9tmd7T0PzNo ratings yet
- 442 FullDocument8 pages442 FullSharly DwijayantiNo ratings yet
- Ajc 1998 81 27Document5 pagesAjc 1998 81 27Satria Bayu PratamaNo ratings yet
- Characterization of Common Measures of Heart Period Variability in Healthy Human Subjects: Implications For Patient MonitoringDocument10 pagesCharacterization of Common Measures of Heart Period Variability in Healthy Human Subjects: Implications For Patient MonitoringJuanGuillermo Acevedo CastilloNo ratings yet
- Effects of Respiratory Interval On Vagal Modulation of Heart RateDocument8 pagesEffects of Respiratory Interval On Vagal Modulation of Heart RateGuillermo Castro ContardoNo ratings yet
- Recurrence Quantification Analysis On Pulse Morphological Changes in Patients With Coronary Heart DiseaseDocument7 pagesRecurrence Quantification Analysis On Pulse Morphological Changes in Patients With Coronary Heart DiseaseArellano M VictorNo ratings yet
- Assessment of Autonomic Functions in Individuals With Cerebral PalsyDocument6 pagesAssessment of Autonomic Functions in Individuals With Cerebral PalsydevdsantoshNo ratings yet
- MilicevicDocument6 pagesMilicevicKlaudijus VitkauskasNo ratings yet
- E A C H R V D: Ffect of Cupressure and Hanges in Eart ATE Ariability IN YsmenorrhoeaDocument6 pagesE A C H R V D: Ffect of Cupressure and Hanges in Eart ATE Ariability IN YsmenorrhoeaPrathibha Lydia Braggs DsouzaNo ratings yet
- Yeshwant 2018Document16 pagesYeshwant 2018StevenSteveIIINo ratings yet
- Cardiac ArrhythmiasDocument5 pagesCardiac Arrhythmiastere glezNo ratings yet
- tmpFD0F TMPDocument9 pagestmpFD0F TMPFrontiersNo ratings yet
- 1 s2.0 S0167527302000311 MainDocument10 pages1 s2.0 S0167527302000311 MainMerve Sibel GungorenNo ratings yet
- PletismografiaDocument10 pagesPletismografiamaxifamous6No ratings yet
- Heart Rate Variability in An Ageing PopulationDocument9 pagesHeart Rate Variability in An Ageing PopulationBasharat BhatNo ratings yet
- Artigo Base SimoneDocument5 pagesArtigo Base SimoneWolfgang ThyerreNo ratings yet
- Heart Rate Variability Reveals Altered Autonomic Regulation in Response To Myocardial Infarction in Experimental AnimalsDocument15 pagesHeart Rate Variability Reveals Altered Autonomic Regulation in Response To Myocardial Infarction in Experimental AnimalsIgnacio BarbieriNo ratings yet
- Ventricular Arrhythmias Underlie Sudden Death in 2Document2 pagesVentricular Arrhythmias Underlie Sudden Death in 2Ganesh B.No ratings yet
- Passive Leg Raising and Fluid Responsiveness Monnet CCM 2006Document6 pagesPassive Leg Raising and Fluid Responsiveness Monnet CCM 2006Leiniker Navarro ReyNo ratings yet
- JcsmJC1900562 W22pod AnsweredDocument13 pagesJcsmJC1900562 W22pod Answeredlion5835No ratings yet
- 2000 Adrenergic and Reflex Abnormalities in Obesity-Related HypertensionDocument5 pages2000 Adrenergic and Reflex Abnormalities in Obesity-Related HypertensionFerroBemNo ratings yet
- 1414 431X BJMBR 51 11 E7704Document8 pages1414 431X BJMBR 51 11 E7704Gede AdiNo ratings yet
- Aksan, G.2014Document9 pagesAksan, G.2014Bogdan HateganNo ratings yet
- Micro-Piezoelectric Pulse Diagnoser and Frequency Domain Analysis of Human Pulse SignalsDocument8 pagesMicro-Piezoelectric Pulse Diagnoser and Frequency Domain Analysis of Human Pulse SignalsAnthrayose ajith chackoNo ratings yet
- Auricchio & Cols. (1999)Document6 pagesAuricchio & Cols. (1999)Luba D'AndreaNo ratings yet
- Guzzetti 2005Document8 pagesGuzzetti 2005Hadj Abdelkader BENGHENIANo ratings yet
- End 2014 0019Document6 pagesEnd 2014 0019Manolin KinNo ratings yet
- Aps 2011118 ADocument6 pagesAps 2011118 ADaniel Lee Eisenberg JacobsNo ratings yet
- Cranial Rhythmic Impulse Related To The Traube-Hering-Mayer OscillationDocument11 pagesCranial Rhythmic Impulse Related To The Traube-Hering-Mayer Oscillationthe_walnutNo ratings yet
- Neuropeptide Y-Immunoreactive Nerve Terminals Parasympathetic Control of The Heart. IIIDocument10 pagesNeuropeptide Y-Immunoreactive Nerve Terminals Parasympathetic Control of The Heart. IIILuammmNo ratings yet
- Tusman 2018Document10 pagesTusman 2018Karel ZertucheNo ratings yet
- R-HRV: An R-Based Software Package For Heart Rate Variability Analysis of ECG RecordingsDocument9 pagesR-HRV: An R-Based Software Package For Heart Rate Variability Analysis of ECG RecordingsFiras ZakNo ratings yet
- An Algorithm For Robust Detection of QRS Onset and Offset in ECG SignalsDocument4 pagesAn Algorithm For Robust Detection of QRS Onset and Offset in ECG SignalsFiras ZakNo ratings yet
- ECM00Document8 pagesECM00Firas ZakNo ratings yet
- Fajloun 2008 Chemical SynthesisDocument5 pagesFajloun 2008 Chemical SynthesisFiras ZakNo ratings yet
- Chap 4 - Detection-ClassificationDocument20 pagesChap 4 - Detection-ClassificationFiras ZakNo ratings yet
- NE5521Document9 pagesNE5521Carlos TibussiNo ratings yet
- Wheelchair Lift Executive SummaryDocument5 pagesWheelchair Lift Executive SummaryIsabelle HanafiahNo ratings yet
- Fire ProtectionDocument44 pagesFire ProtectionAbdul JabbarNo ratings yet
- Qinhuangdao Red Ribbon ParkDocument18 pagesQinhuangdao Red Ribbon Parkjaya saputraNo ratings yet
- S3F94xx BatteryCharger An REV000 060109-0Document40 pagesS3F94xx BatteryCharger An REV000 060109-0Jack ChanNo ratings yet
- EEMUA Calculation ReportDocument3 pagesEEMUA Calculation Reportduf fuNo ratings yet
- Solartech Solar Pumping Inverter: Technical DataDocument1 pageSolartech Solar Pumping Inverter: Technical Dataadolfo escobarNo ratings yet
- Question .Net CoreDocument8 pagesQuestion .Net CorevdshfsdfhsdvNo ratings yet
- Materials Science and Engineering.: Session 9 - The Eutectic Phase DiagramsDocument34 pagesMaterials Science and Engineering.: Session 9 - The Eutectic Phase DiagramsfiestapaganaNo ratings yet
- Axis College of Engineering & Technology, AmbanolyDocument3 pagesAxis College of Engineering & Technology, AmbanolygatewayglobalNo ratings yet
- Civil & Environmental Engineering: Dept. Report October 2002Document40 pagesCivil & Environmental Engineering: Dept. Report October 2002Leonardo HernandezNo ratings yet
- GSM 06 InterfacesDocument17 pagesGSM 06 Interfacesamitcool26No ratings yet
- Zahvalnica Bla BlaDocument2 pagesZahvalnica Bla BlaBiljanaJanjušević100% (1)
- Design and Optimization of An XYZ Parallel Micromanipulator With Flexure HingesDocument26 pagesDesign and Optimization of An XYZ Parallel Micromanipulator With Flexure HingesVijay SakhareNo ratings yet
- Ferroelectric RAM FRAM Seminar Report1Document20 pagesFerroelectric RAM FRAM Seminar Report1Gaurav ReddyNo ratings yet
- A Biometric Model For Examination Screening and Attendance Monitoring in Yaba College of TechnologyDocument6 pagesA Biometric Model For Examination Screening and Attendance Monitoring in Yaba College of TechnologyWorld of Computer Science and Information Technology JournalNo ratings yet
- Fletcher Expanding Tables 2013 v7Document59 pagesFletcher Expanding Tables 2013 v7Silviu Prise100% (1)
- TemperaturaDocument2 pagesTemperaturakamalNo ratings yet
- 40 kVA Specification SheetDocument2 pages40 kVA Specification SheetAlex MohanNo ratings yet
- Kiln Performance - Efficiency FormulasDocument12 pagesKiln Performance - Efficiency FormulasMohamed ZayedNo ratings yet
- Ijesrt: Modelling and Simulation of Solar Photovoltaic Array For Battery Charging Application Using Matlab-SimulinkDocument5 pagesIjesrt: Modelling and Simulation of Solar Photovoltaic Array For Battery Charging Application Using Matlab-Simulinksrinureddy2014No ratings yet
- 5950 Part 1-1990Document120 pages5950 Part 1-1990José Miguel100% (1)
- How To Install Openbravo ERP On Debian LennyDocument4 pagesHow To Install Openbravo ERP On Debian LennyfromemNo ratings yet
- ASAP MethodologyDocument14 pagesASAP MethodologyTiffany HughesNo ratings yet
- Saskatchewan Drivers HandbookDocument175 pagesSaskatchewan Drivers HandbookdrivershandbooksNo ratings yet
- WPH02 01 Que 20160120Document24 pagesWPH02 01 Que 20160120Omar HashemNo ratings yet
- Pvu-L0880er GaDocument1 pagePvu-L0880er GaarunghandwalNo ratings yet
- 3-Oracle Application Framework (OAF) Training Guide - EO, VO, Page, Query Region, LOV, PPRDocument73 pages3-Oracle Application Framework (OAF) Training Guide - EO, VO, Page, Query Region, LOV, PPRPreethi KishoreNo ratings yet