Professional Documents
Culture Documents
24 Acid-Base Titration
Uploaded by
gardarr11Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
24 Acid-Base Titration
Uploaded by
gardarr11Copyright:
Available Formats
Computer
Acid-Base Titration
24
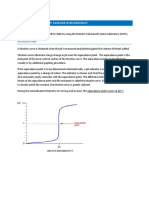
A titration is a process used to determine the volume of a solution needed to react with a given amount of another substance. In this experiment, you will titrate hydrochloric acid solution, HCl, with a basic sodium hydroxide solution, NaOH. The concentration of the NaOH solution is given and you will determine the unknown concentration of the HCl. Hydrogen ions from the HCl react with hydroxide ions from the NaOH in a one-to-one ratio to produce water in the overall reaction: H+(aq) + Cl(aq) + Na+(aq) +OH(aq) H2O(l) + Na+(aq) + Cl(aq) When an HCl solution is titrated with an NaOH solution, the pH of the acidic solution is initially low. As base is added, the change in pH is quite gradual until close to the equivalence point, when equimolar amounts of acid and base have been mixed. Near the equivalence point, the pH increases very rapidly, as shown in Figure 1. The change in pH then becomes more gradual again, before leveling off with the addition of excess base. In this experiment, you will use a computer to monitor pH as you titrate. The region of most rapid pH change will then be used to determine the equivalence point. The volume of NaOH titrant used at the equivalence point will be used to determine the molarity of the HCl.
Figure 1
OBJECTIVES
In this experiment, you will pH Sensor to monitor changes in pH as sodium hydroxide solution is added to a hydrochloric acid solution. Plot a graph of pH vs. volume of sodium hydroxide solution added. Use the graph to determine the equivalence point of the titration. Use the results to calculate the concentration of the hydrochloric acid solution.
Use a
Chemistry with Vernier
24 - 1
Computer 24
MATERIALS
Materials for both Method 1 (buret) and Method 2 (Drop Counter)
computer Vernier computer interface Logger Pro Vernier pH Sensor HCl solution, unknown concentration ~0.1 M NaOH solution pipet bulb or pump
Materials required only for Method 1 (buret)
magnetic stirrer (if available) stirring bar or Microstirrer (if available) wash bottle distilled water ring stand 1 utility clamp 250 mL beaker 2nd utility clamp 2nd 250 mL beaker 100 mL beaker 10 mL graduated cylinder
50 mL buret 10 mL pipet Vernier Drop Counter 60 mL reagent reservoir 5 mL pipet or graduated 10 mL pipet
Materials required only for Method 2 (Drop Counter)
CHOOSING A METHOD
Method 1 has the student deliver volumes of NaOH titrant from a buret. After titrant is added, and pH values have stabilized, the student is prompted to enter the buret reading manually and a pH-volume data pair is stored. Method 2 uses a Vernier Drop Counter to take volume readings. NaOH titrant is delivered drop by drop from the reagent reservoir through the Drop Counter slot. After the drop reacts with the reagent in the beaker, the volume of the drop is calculated, and a pH-volume data pair is stored.
METHOD 1: Measuring Volume Using a Buret
1. Obtain and wear goggles. 2. Add 50 mL of distilled water to a 250 mL beaker. Use a pipet bulb (or pipet pump) to pipet 10.0 mL of the HCl solution into the distilled water in the 250 mL beaker. CAUTION: Handle the hydrochloric acid with care. It can cause painful burns if it comes in contact with the skin. 3. Place the beaker on a magnetic stirrer and add a stirring bar. If no magnetic stirrer is available, you need to stir with a stirring rod during the titration. 4. Use a utility clamp to suspend a pH Sensor on a ring stand as shown here. Position the pH Sensor in the HCl solution and adjust its position so it will not be struck by the stirring bar. Turn on the magnetic stirrer, and adjust it to a medium stirring rate (with no splashing of solution). 5. Obtain approximately 60 mL of ~0.1 M NaOH solution in a 250 mL beaker. Obtain a 50 mL buret and rinse the buret with a few mL of the ~0.1 M NaOH solution. Use a utility clamp to attach
24 - 2
Chemistry with Vernier
Acid-Base Titration the buret to the ring stand as shown here. Fill the buret a little above the 0.00 mL level of the buret with ~0.1 M NaOH solution. Drain a small amount of NaOH solution into the beaker so it fills the buret tip and leaves the NaOH at the 0.00 mL level of the buret. Record the precise concentration of the NaOH solution in your data table. Dispose of the waste solution from this step as directed by your teacher. CAUTION: Sodium hydroxide solution is caustic. Avoid spilling it on your skin or clothing. 6. Connect the pH Sensor to the computer interface. Prepare the computer for data collection by opening the file 24a Acid-Base Titration from the Chemistry with Vernier folder. Check to see that the pH value is between 2 and 3. 7. Before adding NaOH titrant, click and monitor pH for 5-10 seconds. Once the displayed pH reading has stabilized, click . In the edit box, type 0 (for 0 mL added). Press the ENTER key or click to store the first data pair for this experiment. 8. You are now ready to begin the titration. This process goes faster if one person manipulates and reads the buret while another person operates the computer and enters volumes. a. Add the next increment of NaOH titrant (enough to raise the pH about 0.15 units). When the pH stabilizes, again click . In the edit box, type the current buret reading, to the nearest 0.01 mL. Press ENTER or click . You have now saved the second data pair for the experiment. b. Continue adding NaOH solution in increments that raise the pH by about 0.15 units and enter the buret reading after each increment. Proceed in this manner until the pH is 3.5. c. When a pH value of approximately 3.5 is reached, change to a one-drop increment. Enter a new buret reading after each increment. Note: It is important that all increment volumes in this part of the titration be equal; that is, one-drop increments. d. After a pH value of approximately 10 is reached, again add larger increments that raise the pH by about 0.15 pH units, and enter the buret level after each increment. e. Continue adding NaOH solution until the pH value remains constant. 9. When you have finished collecting data, click directed by your teacher. 10. Print copies of the table and the graph. 11. If time permits, repeat the procedure. . Dispose of the beaker contents as
PROCESSING THE DATA
1. Use your graph and data table to determine the volume of NaOH titrant used in each trial. Examine the data to find the largest increase in pH values upon the addition of 1 drop of NaOH solution. Find and record the NaOH volume just before and after this jump. 2. Determine the volume of NaOH added at the equivalence point. To do this, add the two NaOH values determined above and divide by two. 3. Calculate the number of moles of NaOH used. 4. Using the equation for the neutralization reaction given in the introduction, determine the number of moles of HCl used.
Chemistry with Vernier
24 - 3
Computer 24 5. Calculate the HCl concentration using the volume of unknown HCl you pipeted out for each titration. 6. (Optional) If you did two titrations, determine the average [HCl] in mol/L.
DATA TABLE
Concentration of NaOH NaOH volume added before the largest pH increase NaOH volume added after the largest pH increase Volume of NaOH added at equivalence point 0.1M 15mL 17mL M mL mL
17mL Moles NaOH
mL
5.88mol Moles HCl 60mL, pH=2.27 .00537mol/L
mol
.0895mol Concentration of HCl 0.0895mol / 10mL
mol
8.95M Average [HCl]
mol/L
24 - 4
Chemistry with Vernier
Acid-Base Titration
EQUIVALENCE POINT DETERMINATION: An Alternate Method
An alternate way of determining the precise equivalence point of the titration is to take the first and second derivatives of the pH-volume data. The equivalence point volume corresponds to the peak (maximum) value of the first derivative plot, and to the volume where the second derivative equals zero on the second derivative plot. 1. In Method 1, view the first-derivative graph ( pH/vol) by clicking the on the vertical-axis label (pH), and choose First Derivative. You may need to autoscale the new graph by clicking the Autoscale button, . In Method 2, you can also view the first derivative graph (pH/vol) on Page 2 of the experiment file by clicking the Next Page button, . On Page 2 you will see a plot of first derivative vs. volume. 2. In Method 1, view the second-derivative graph (2pH/vol2) by clicking on the vertical-axis label, and choosing Second Derivative. In Method 2, view the second-derivative on Page 3 by clicking the Next Page button, .
Chemistry with Vernier
24 - 5
You might also like
- CWV 24 COMP Acid - Base - Titration PDFDocument8 pagesCWV 24 COMP Acid - Base - Titration PDFTha KantanaNo ratings yet
- Titration of A Diprotic Acid Identifying An Unknown: ObjectiveDocument9 pagesTitration of A Diprotic Acid Identifying An Unknown: ObjectivePuji WulandariNo ratings yet
- Acid-Base Titration by Dan HolmquistDocument5 pagesAcid-Base Titration by Dan HolmquistPaul SchumannNo ratings yet
- CWV 35 COMP Phosphoric - Acid PDFDocument4 pagesCWV 35 COMP Phosphoric - Acid PDFNaveen KumarNo ratings yet
- Acid - Base Lab 2016 BegleyDocument7 pagesAcid - Base Lab 2016 BegleyIsaac SnitkoffNo ratings yet
- CHEM A 24 COMP Half TitrationDocument4 pagesCHEM A 24 COMP Half TitrationSung Hoon ParkNo ratings yet
- CHEM I 17 Acid Base Titrations OpenDocument4 pagesCHEM I 17 Acid Base Titrations OpenAchinthya PereraNo ratings yet
- 25 Titration Diprotic AcidDocument8 pages25 Titration Diprotic AcidAngel DediosNo ratings yet
- Practical 4Document2 pagesPractical 4vimukthi gunasinghaNo ratings yet
- Titration of A Diprotic Acid: Identifying An Unknown by Dan HolmquistDocument8 pagesTitration of A Diprotic Acid: Identifying An Unknown by Dan HolmquistPaul Schumann0% (1)
- Titration Diprotic AcidDocument9 pagesTitration Diprotic AcidjaNo ratings yet
- Part I: Titration With An Indicator: Data and ObservationsDocument5 pagesPart I: Titration With An Indicator: Data and ObservationsjiNo ratings yet
- 62 Experiment #5. Titration of An Acid Using A PH MeterDocument7 pages62 Experiment #5. Titration of An Acid Using A PH MeteryumnatehreemNo ratings yet
- PH Meters Purdue University Instrument Van Project Acid-Base Titration Using A PH MeterDocument5 pagesPH Meters Purdue University Instrument Van Project Acid-Base Titration Using A PH MeterNatsu PatnaikNo ratings yet
- Exp2 - A-B Titration - w2013-2Document10 pagesExp2 - A-B Titration - w2013-2lovingbubblesNo ratings yet
- Lab R.2 - Concentration of An Acid - Three WaysDocument6 pagesLab R.2 - Concentration of An Acid - Three WaysAdarsh Raj TiwariNo ratings yet
- Sulfamic Acid Titration C12!5!10Document5 pagesSulfamic Acid Titration C12!5!10Anonymous 1gXoNDYcNo ratings yet
- Weak Acid Strong Base Titration LabDocument8 pagesWeak Acid Strong Base Titration Labapi-265089380100% (1)
- PH Titration LabQuest PDFDocument3 pagesPH Titration LabQuest PDFAntónio MatiasNo ratings yet
- 7B Acid-Base Titration - Unknown HCLDocument1 page7B Acid-Base Titration - Unknown HCLJacob DaughertyNo ratings yet
- Experiment 6 Titration II - Acid Dissociation ConstantDocument8 pagesExperiment 6 Titration II - Acid Dissociation ConstantPanneer SelvamNo ratings yet
- Mixture of Carbonate BicarbonateDocument9 pagesMixture of Carbonate BicarbonateIan Justine SanchezNo ratings yet
- S Y PH MeterDocument2 pagesS Y PH MeterNeelam KapoorNo ratings yet
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesDocument5 pagesAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- 06 and 07 Standardization of NaOH and Acid Base TitrationDocument16 pages06 and 07 Standardization of NaOH and Acid Base TitrationTyler Hardy80% (5)
- Lab 11 Acids, Bases, PH, Hydrolysis, and BuffersDocument10 pagesLab 11 Acids, Bases, PH, Hydrolysis, and BuffersChing Wai Yong67% (3)
- Potentiometric Titration Ex17Document10 pagesPotentiometric Titration Ex17Tien HaminhNo ratings yet
- Acetic Acid Dissociation Constant S11Document7 pagesAcetic Acid Dissociation Constant S11Ayesha ShahidNo ratings yet
- Chemistry Report - Titration of VinegarDocument7 pagesChemistry Report - Titration of VinegarSabestNo ratings yet
- Buffers Lab Write UpDocument7 pagesBuffers Lab Write Upapi-20078641850% (2)
- Acid Base TitrationDocument6 pagesAcid Base TitrationSyameer YusofNo ratings yet
- Lab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, KaDocument10 pagesLab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, Kaenock yegonNo ratings yet
- Sinha TitrationcurvesDocument11 pagesSinha TitrationcurvesRadu StafiNo ratings yet
- Acid and Base Titrations Using A PH MeterDocument5 pagesAcid and Base Titrations Using A PH MeterJason Oliver ChanNo ratings yet
- Experiment 1&2Document8 pagesExperiment 1&2Fatima AhmedNo ratings yet
- Summary: Phthalate (KHP) Solution Which The Molarity Is Already Known. Expressing The Chemical ReactionDocument15 pagesSummary: Phthalate (KHP) Solution Which The Molarity Is Already Known. Expressing The Chemical ReactionDayledaniel SorvetoNo ratings yet
- 111 TitrationDocument12 pages111 TitrationElisaNo ratings yet
- Lab Experiment 3 - PH TitrationDocument1 pageLab Experiment 3 - PH Titrationfive shadowsNo ratings yet
- Determination of Acetic Acid in VinegarDocument6 pagesDetermination of Acetic Acid in VinegarTishko0% (1)
- Acid - Base Titration: Reaction of Naoh With HCLDocument6 pagesAcid - Base Titration: Reaction of Naoh With HCLAcamlee AriffinNo ratings yet
- Exp 6 PH Metric TitrationDocument3 pagesExp 6 PH Metric TitrationDeep DaveNo ratings yet
- Preparing Buffers and Buffer CapacityDocument7 pagesPreparing Buffers and Buffer CapacityAndreaKatovic100% (1)
- Determination Acetic AcidDocument21 pagesDetermination Acetic Acidameyakem100% (1)
- S Determination of Phosphoric Acid Content in SoftdrinksDocument5 pagesS Determination of Phosphoric Acid Content in SoftdrinksMike Anderson0% (1)
- 07.03 PH Lab ReportDocument4 pages07.03 PH Lab ReportBladeNo ratings yet
- Jce 2007 P 0124 WDocument25 pagesJce 2007 P 0124 WAlexaNo ratings yet
- Quantitative Reactions and Titrations ExperimentDocument5 pagesQuantitative Reactions and Titrations ExperimentJeremy BarrettNo ratings yet
- Lab Format:: Lab 2: Determination of Carbonate/BicarbonateDocument5 pagesLab Format:: Lab 2: Determination of Carbonate/BicarbonateAnaya FatimaNo ratings yet
- Titration of A Poliprotic AcidDocument7 pagesTitration of A Poliprotic AcidRaduNo ratings yet
- How To Measure The PHPZC Using The PH Drift MethodDocument2 pagesHow To Measure The PHPZC Using The PH Drift Methodpaola aldana100% (1)
- Titration Notes: MethodDocument3 pagesTitration Notes: MethodArSlanRahatNo ratings yet
- Potentiometric Titration CurvesDocument5 pagesPotentiometric Titration CurvesDavid GrahamNo ratings yet
- 1Document8 pages1Isma WantiNo ratings yet
- Experiment 4 Preparation of Standardized SolutionsDocument10 pagesExperiment 4 Preparation of Standardized SolutionsJohn Dy100% (1)
- Titration LabDocument8 pagesTitration LabFarhan HabibzaiNo ratings yet
- Tit RationDocument7 pagesTit RationgautamahujaNo ratings yet
- GA7 Potentio Titr Rev7 99Document9 pagesGA7 Potentio Titr Rev7 99Jerome SadudaquilNo ratings yet
- Experiment 2Document6 pagesExperiment 2Raj PatelNo ratings yet
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- Different DepartmentsDocument13 pagesDifferent DepartmentsChiru GoudaNo ratings yet
- Monthly Chemical Report - 2018Document28 pagesMonthly Chemical Report - 2018Abdelrahman MaherNo ratings yet
- Sample Test Paper For Class & (Science)Document4 pagesSample Test Paper For Class & (Science)Surya SalariaNo ratings yet
- Hydrochloric AcidDocument6 pagesHydrochloric AcidKhoirul HudaNo ratings yet
- Company Profile: PT Mitra Agung SejatiDocument12 pagesCompany Profile: PT Mitra Agung SejatiDimas Theo PNo ratings yet
- Guideline Standard Preparation AAS enDocument8 pagesGuideline Standard Preparation AAS enwildan abiyyiNo ratings yet
- Chemistry: Edexcel GCEDocument24 pagesChemistry: Edexcel GCEaquu174015100% (1)
- 2Mg+O - 2mgo Mgo+H - MG (Oh) Turns Blue, Indicating It'S A Basic Solution. No ChangeDocument3 pages2Mg+O - 2mgo Mgo+H - MG (Oh) Turns Blue, Indicating It'S A Basic Solution. No ChangeDaenizeeNo ratings yet
- Acids, Bases and Salts MCQS: (D) (Ii) and (Iv)Document12 pagesAcids, Bases and Salts MCQS: (D) (Ii) and (Iv)Atharva BhokareNo ratings yet
- Sodamint PDFDocument5 pagesSodamint PDFalwi firdausNo ratings yet
- Measurement of Thin Chromium Coatings by Spot Test: Standard Guide ForDocument3 pagesMeasurement of Thin Chromium Coatings by Spot Test: Standard Guide ForTuanbk NguyenNo ratings yet
- Paper 2 QP 2017-2022Document585 pagesPaper 2 QP 2017-2022Farrah IezzahNo ratings yet
- BAM S08 12 Sulphates Dec97Document7 pagesBAM S08 12 Sulphates Dec97DhileepNo ratings yet
- College of Dunaújváros Metallurgy: Report 2Document5 pagesCollege of Dunaújváros Metallurgy: Report 2Luiz Gustavo Gomes NoceraNo ratings yet
- Systematic Analysis of Simple Salt-7Document7 pagesSystematic Analysis of Simple Salt-7Bala Murugan.VNo ratings yet
- Ojt Narative Report in Seda HotelDocument59 pagesOjt Narative Report in Seda HotelGlyzel Escrimadora50% (2)
- Reaction of Sodium Thiosulphate and Hydrochloric AcidDocument5 pagesReaction of Sodium Thiosulphate and Hydrochloric Acidapi-373250088% (8)
- Materials Science in Semiconductor ProcessingDocument5 pagesMaterials Science in Semiconductor ProcessingYasmine JaziriNo ratings yet
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument35 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsLOLA PATRICIA MORALES DE LA CUBANo ratings yet
- Practical Boiler Water TreatmentDocument285 pagesPractical Boiler Water TreatmentLuciano Cardoso Vasconcelos100% (14)
- Syllabus For Preboard - 2 Class XDocument7 pagesSyllabus For Preboard - 2 Class XKriday MisriNo ratings yet
- Cambridge IGCSE: CHEMISTRY 0620/61Document12 pagesCambridge IGCSE: CHEMISTRY 0620/61SasukeNo ratings yet
- Preparation of PH Buffer Solutions (AnalChem Resources, Ex Web10!04!1613!08!07pdf)Document5 pagesPreparation of PH Buffer Solutions (AnalChem Resources, Ex Web10!04!1613!08!07pdf)Diana ChiscaNo ratings yet
- Harold V Refining GoldDocument34 pagesHarold V Refining GoldAFLAC ............100% (3)
- 4mmc SynthesisDocument2 pages4mmc Synthesisivan volkovNo ratings yet
- 14 Matrix Acidizing of SandstonesDocument24 pages14 Matrix Acidizing of SandstoneslapinNo ratings yet
- D 1726 - 90 r96 - Rde3mjytukveDocument5 pagesD 1726 - 90 r96 - Rde3mjytukvealienz1988newNo ratings yet
- Extraction and Characterization of Pectin From Saba Banana (Musa 'Saba' (Musa Acuminata X Musa Balbisiana) ) Peel Wastes: A Preliminary StudyDocument7 pagesExtraction and Characterization of Pectin From Saba Banana (Musa 'Saba' (Musa Acuminata X Musa Balbisiana) ) Peel Wastes: A Preliminary StudyRhenz Jarren CarreonNo ratings yet
- General Methods Boiling Point and Distillation RangeDocument83 pagesGeneral Methods Boiling Point and Distillation RangeAnonymous MVHQ97KEoPNo ratings yet
- Process Calculation Rev-1Document18 pagesProcess Calculation Rev-1govindharajalu100% (1)