Professional Documents
Culture Documents
Pyogenic Liver Abscess
Uploaded by
Patcharaporn Fern KlongklaewOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pyogenic Liver Abscess
Uploaded by
Patcharaporn Fern KlongklaewCopyright:
Available Formats
Pyogenic liver abscess
Authors Joshua Davis, MBBS, FRACP Malcolm McDonald, PhD, FRACP, FRCPA Section Editor Stephen B Calderwood, MD Deputy Editor Allyson Bloom, MD Disclosures All topics are updated as new evidence becomes available and our peer review process is complete. Literature review current through: Feb 2012. | This topic last updated: .. 18, 2011. INTRODUCTION Pyogenic liver abscesses usually develop following peritonitis due to leakage of intraabdominal bowel contents that subsequently spread to liver via the portal circulation or via direct spread from biliary infection. They may also result from arterial hematogenous seeding in the setting of systemic infection. The clinical approach to pyogenic liver abscess will be reviewed here. Amebic abscesses are discussed separately. (See "Extraintestinal Entamoeba histolytica amebiasis".) EPIDEMIOLOGY Liver abscesses are the most common type of visceral abscess; in a report of 540 cases of intraabdominal abscesses, pyogenic liver abscesses accounted for 48 percent of visceral abscesses and 13 percent of intraabdominal abscesses [1]. The annual incidence of liver abscess has been estimated at 2.3 cases per 100,000 population and is higher among men than women (3.3 versus 1.3 per 100,000) [2-4]; substantially higher rates have been reported in Taiwan (17.6 cases per 100,000) [5]. Risk factors include diabetes, underlying hepatobiliary or pancreatic disease, and liver transplant [2,3,6,7]. Geographic and host factors may also play a role; for example, a primary invasive liver abscess syndrome due to K. pneumoniae has been described in East Asia. This is discussed separately. (See "Invasive liver abscess syndrome caused by Klebsiella pneumoniae".) The mortality rate in developed countries ranges from 2 to 12 percent [3,8]. Independent risk factors for mortality include need for open surgical drainage, the presence of malignancy, and the presence of anaerobic infection [9,10]. PATHOGENESIS A considerable proportion of pyogenic liver abscesses follow one or more episodes of portal vein pyemia, usually related to bowel leakage and peritonitis. Another important route is direct spread from biliary infection. Underlying biliary tract disease such as gallstones or malignant obstruction is present in 40 to 60 percent of cases [2,8,11]. Occasionally, abscesses arise from surgical or penetrating wounds [12]. Liver abscesses may also result from hematogenous seeding from the systemic circulation. A monomicrobial liver abscess due to a streptococcal or staphylococcal species should prompt evaluation for an additional source of infection. Liver abscesses most commonly involve the right lobe of the liver, probably because it is larger and has greater blood supply than the left and caudate lobes. Liver abscess may also be accompanied by pylephlebitis [13]. (See "Pylephlebitis".) MICROBIOLOGY Many pathogens have been described; this variability reflects the different causes, types of medical intervention (such as biliary tree stenting, or immunosuppression due to
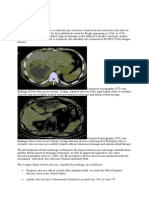
cancer chemotherapy) and geographic differences. Most pyogenic liver abscesses are polymicrobial; mixed enteric facultative and anaerobic species are the most common pathogens. Anaerobes are probably under-reported because they are difficult to culture and characterize in the laboratory. For example, in one series of 233 cases, mixed facultative and anaerobic species were implicated in onethird of patients, and bacteremia was documented in 56 percent of cases [2]. The highly variable microbiology justifies pursuing a microbiological diagnosis in virtually every case. Potential pathogens include: The Streptococcus milleri or S. anginosus group (including S. constellatus and S. intermedius) is an important cause of liver abscess. When implicated, it should prompt a search for simultaneous metastatic infections at other locations. (See "Infections due to the Streptococcus anginosus group".) S. aureus, S. pyogenes, and other Gram positive cocci are recognized pathogens in specific circumstances [14]. For example, in a report of liver abscesses in patients who underwent transarterial embolization for hepatocellular carcinoma, they accounted for 60 percent of pathogens. (See "Nonsurgical therapies for localized hepatocellular carcinoma: Transarterial embolization, radiotherapy, and radioembolization", section on 'Complications'.) Candida species have also been implicated in pyogenic liver abscess and accounted for 22 percent of liver abscesses in one series [2]. Hepatosplenic candidiasis can occur in patients who have received chemotherapy and presents with recovery of neutrophil counts following a neutropenic episode. (See "Hepatosplenic candidiasis (chronic disseminated candidiasis)".) Klebsiella pneumoniae is an important emerging pathogen. This syndrome is discussed in detail separately (see "Invasive liver abscess syndrome caused by Klebsiella pneumoniae"). Tuberculous liver abscesses are uncommon but should be considered when typical pyogenic organisms are not recovered from cultures [15,16]. (See "Clinical manifestations, diagnosis, and treatment of miliary tuberculosis".) Burkholderia pseudomallei (the agent of Melioidosis) should be considered in patients from endemic areas (Southeast Asia and Northern Australia). (See "Epidemiology, pathogenesis, clinical manifestations, and diagnosis of melioidosis".) Amebiasis should be considered as a cause of primary liver abscess, especially in patients who are from or have traveled to an endemic area within the past six months. The clinical course and appearance may be difficult to distinguish from pyogenic liver abscess; this is discussed in detail separately. (See "Extraintestinal Entamoeba histolytica amebiasis".) CLINICAL MANIFESTATIONS The typical clinical manifestations of pyogenic liver abscess are fever and abdominal pain. Other common symptoms include nausea, vomiting, anorexia, weight loss, and malaise. Fever occurs in approximately 90 percent of patients, and abdominal symptoms occur in 50 to 75 percent of patients [2,3,8,17]. Abdominal symptoms and signs are usually localized to the right upper quadrant and may include pain, guarding, rocking tenderness (pain caused by gently rocking the patient's abdomen), and even rebound tenderness. About one-half of patients with liver abscess have hepatomegaly, right upper quadrant tenderness, or jaundice [17]. The absence of right upper quadrant findings does not exclude liver abscess. DIAGNOSIS The diagnosis of pyogenic liver abscess is made by history, clinical examination, and radiographic imaging followed by aspiration and culture of the abscess material. Imaging Computed tomography (CT) scan and ultrasound are the modalities of choice (picture 1) [12]. CT usually shows a fluid collection with surrounding edema. There may also be stranding and loculated subcollections. Pyogenic liver abscess cannot be reliably distinguished from amebic abscess
by imaging studies [15]. (See "Extraintestinal Entamoeba histolytica amebiasis", section on 'Amebic liver abscess'.) Abscesses must be distinguished from tumors and cysts. Tumors have a solid radiographic appearance and may contain areas of calcification. Necrosis and bleeding within a tumor may lead to a fluid-filled appearance; in such circumstances radiographic differentiation from abscess can be challenging. Cysts appear as fluid collections without surrounding stranding or hyperemia. An elevated right hemidiaphragm, right basilar infiltrate, or right-sided pleural effusion can be seen in 25 to 35 percent of cases [18]; these findings should prompt further investigation of the right upper quadrant with CT or ultrasonography. Magnetic resonance imaging and tagged white blood cell scans are less useful for distinguishing abscess from other causes of liver mass. Microbial cultures Material obtained from CT or ultrasound-guided aspiration should be sent to the laboratory for gram stain and culture (both aerobic and anaerobic). Anaerobic culture should be specifically requested on the laboratory requisition. Blood cultures are essential; they are positive in up to 50 percent of cases [19]. Cultures obtained from existing drains are NOT adequate for guiding antimicrobial therapy, since they are often contaminated with skin flora and other organisms. This was demonstrated in a study of 66 cases of liver abscess; cultures results obtained via radiographic guidance were compared with cultures obtained from a drain that had been in place for at least 48 hours [16]. Cultures from percutaneous specimens correlated with cultures from drainage catheters in only one-half of cases. Treatment based upon drainage culture results alone would have led to inappropriate therapy for the remaining patients. Laboratory findings Laboratory abnormalities may include elevated bilirubin and/or liver enzymes. Serum alkaline phosphatase is elevated in 67 to 90 percent of cases and serum bilirubin and aspartate aminotransferase concentrations are elevated in about one-half of cases [2,8,17]. Other laboratory abnormalities may include leukocytosis, hypoalbuminemia, and anemia (normochromic, normocytic). TREATMENT Treatment of pyogenic liver abscess should include drainage and antibiotic therapy. Drainage Drainage techniques include CT-guided or ultrasound-guided percutaneous drainage (with or without catheter placement), surgical drainage, or drainage by endoscopic retrograde cholangiopancreatography (ERCP). For single abscesses with a diameter <5 cm, either percutaneous catheter drainage or needle aspiration is acceptable [20-23]. Drainage catheters should remain in place until drainage is minimal (usually up to seven days). Repeat needle aspiration may be required in up to half of cases if a catheter is not left in situ [20,21]. For percutaneous management of single abscesses with diameter >5 cm, catheter drainage is preferred over needle aspiration. These principles were illustrated in a trial of 60 patients with pyogenic liver abscess treated with antibiotics and percutaneous drainage via catheter or needle aspiration [23]. Among patients with an abscess diameter >5 cm, treatment was successful in 100 percent of patients treated with catheter drainage compared with 50 percent of patients with needle aspiration. Successful outcomes were observed for all patients with abscess ?5 cm, regardless of drainage modality.
For single abscesses with diameter >5 cm, some favor surgical intervention over percutaneous drainage [24,25]. The efficacy of this approach was suggested in a retrospective study of 80 patients with abscess >5 cm managed with percutaneous or surgical drainage; there was no difference in mortality, morbidity, duration of fever or complication rates. However, the rate of treatment failure was lower with surgical drainage (7 versus 28 percent). Surgical drainage is also appropriate in the following circumstances: Multiple abscesses Loculated abscesses Abscesses with viscous contents obstructing the drainage catheter Underlying disease requiring primary surgical management Inadequate response to percutaneous drainage within seven days Multiple or loculated abscesses may be successfully managed by percutaneous drainage; this was illustrated in a retrospective study of patients with pyogenic liver abscess [26]. Successful percutaneous drainage was achieved in the setting of multiple abscesses (22 of 24 patients) and multiloculated abscesses (51 of 54 patients) [26]. Endoscopic retrograde cholangiopancreatography (ERCP) can be useful for drainage of liver abscesses in patients with previous biliary procedures whose infection communicates with the biliary tree [11,27]. Antibiotics No randomized controlled trials have evaluated empiric antibiotic regimens for treatment of pyogenic liver abscess. Treatment recommendations are based upon the probable source of infection and should be guided by local bacterial resistance patterns, if known. (See 'Microbiology' above.) Empiric broad-spectrum parenteral antibiotics should be administered pending abscess gram stain and culture results. We suggest one of the regimens outlined in the table (table 1). Regardless of the initial empiric regimen, the therapeutic regimen should be revisited once culture and susceptibility results are available. Recovery of more than one organism should suggest polymicrobial infection including anaerobes, even if no anaerobes are isolated in culture. In such circumstances, anaerobic coverage should be continued. Duration of therapy There are no randomized controlled trials evaluating the optimal duration of therapy. This is typically determined by the extent of infection and the patient's clinical response to initial management. Patients with abscess(es) that are difficult to drain or slow to resolve on followup imaging usually require longer courses of therapy. Useful clinical indicators to follow are temperature, white blood cell count and serum C-reactive protein. Follow-up imaging should only be performed in the setting of persistent clinical symptoms or if drainage is not proceeding as expected; radiological abnormalities resolve much more slowly than clinical and biochemical markers. Among 102 pyogenic liver abscess patients in Nepal, the mean time to ultrasonographic resolution of abscesses <10 cm was 16 weeks; mean time to resolution for abscesses >10 cm was 22 weeks [28].
Drainage catheters should remain in place until drainage is minimal (usually up to seven days). If percutaneous needle aspiration was performed without catheter placement, repeat aspiration may be required in up to one-half of cases [20,21]. Antibiotic therapy should be continued for four to six weeks [29]. Patients who have had a good response to initial drainage should be treated with two to four weeks of parenteral therapy, while patients with incomplete drainage should receive four to six weeks of parenteral therapy. The remainder of the course can then be completed with oral therapy tailored to culture results [22,23]. If culture results are not available, reasonable empiric oral antibiotic choices include amoxicillinclavulanate alone or a fluoroquinolone (ciprofloxacin or levofloxacin) plus metronidazole. SUMMARY AND RECOMMENDATIONS The clinical manifestations of pyogenic liver abscess usually include fever and abdominal pain; other symptoms may include nausea, vomiting, anorexia, weight loss and malaise. (See 'Clinical manifestations' above.) The diagnosis of pyogenic liver abscess is confirmed by radiographic imaging (computed tomography or ultrasound) followed by aspiration and culture of the abscess material. (See 'Diagnosis' above.) Most pyogenic liver abscesses are polymicrobial. Amebic abscess is best distinguished from pyogenic liver abscess by serology. (See 'Microbiology' above.) We recommend draining liver abscesses (Grade 1B). For drainage of abscesses ?5 cm in diameter, we suggest needle aspiration rather than percutaneous catheter drainage (Grade 2C). Repeat needle aspiration may be required. (See 'Drainage' above.) For drainage of abscesses >5 cm in diameter, we suggest percutaneous catheter drainage rather than needle aspiration (Grade 2B). Drainage catheters should remain in place until drainage ceases (usually up to seven days). (See 'Drainage' above.) We suggest surgical drainage (rather than percutaneous drainage) in the following circumstances (Grade 2C). (See 'Drainage' above.): Multiple abscesses (depending on number, position, and size) Loculated abscesses Abscesses with viscous contents obstructing drainage catheter Underlying disease requiring primary surgical management Inadequate response to percutaneous drainage within seven days We recommend empiric parenteral antibiotic therapy pending culture and susceptibility results (Grade 1C). Suggested regimens are outlined in the table (table 1). (See 'Antibiotics' above.) When there is a clinical response to therapy, oral antibiotics may be substituted for parenteral therapy (with the guidance of susceptibility testing) to complete a four to six week course of treatment. (See 'Duration of therapy' above.) Use of UpToDate is subject to the Subscription and License Agreement. REFERENCES
You might also like
- Icru 83Document112 pagesIcru 83HanKethyanethNo ratings yet
- Colangitis Esclerosante Secundaria 2013Document9 pagesColangitis Esclerosante Secundaria 2013Lili AlcarazNo ratings yet
- 3D OcclusogramsDocument9 pages3D OcclusogramsRobert Eustaquio100% (1)
- Revolution™ EVO: Technical Reference ManualDocument9 pagesRevolution™ EVO: Technical Reference ManualCeoĐứcTrườngNo ratings yet
- Screeners examination test questions and abbreviationsDocument47 pagesScreeners examination test questions and abbreviationsSubash Ezhumalai93% (40)
- Comp ReDocument15 pagesComp ReROBERT C. REÑA, BSN, RN, MAN (ue)No ratings yet
- Pyogenic Liver AbscessDocument10 pagesPyogenic Liver AbscessErnesto Sebastian GarcíaNo ratings yet
- LIVER ABSCESS GUIDEDocument10 pagesLIVER ABSCESS GUIDEFergie Erazo BeltránNo ratings yet
- Absceso Hepatico 2014 InglesDocument11 pagesAbsceso Hepatico 2014 InglesYeison SierraNo ratings yet
- CholangitisDocument11 pagesCholangitisNilamsari KurniasihNo ratings yet
- There Are Many Potential Causes of Liver AbscessesDocument4 pagesThere Are Many Potential Causes of Liver AbscessesmarianmadhurNo ratings yet
- Chylous, Bloody, and Pancreatic Ascites - UpToDateDocument26 pagesChylous, Bloody, and Pancreatic Ascites - UpToDateAndrea RodríguezNo ratings yet
- 2017 UpToDateDocument13 pages2017 UpToDateThiago HübnerNo ratings yet
- Kista LiverDocument16 pagesKista LiverInomy ClaudiaNo ratings yet
- DefaultDocument55 pagesDefaultt228j4mdw4No ratings yet
- Liver Abscess Guide: Symptoms, Causes, Diagnosis and TreatmentDocument17 pagesLiver Abscess Guide: Symptoms, Causes, Diagnosis and TreatmentRahmat HidayatNo ratings yet
- Liver AbscessDocument3 pagesLiver AbscessLyiuiu TranNo ratings yet
- Empiema Kandung EmpeduDocument7 pagesEmpiema Kandung EmpeduHasya KinasihNo ratings yet
- Case Alcohol Abuse and Unusual Abdominal Pain in A 49-Year-OldDocument7 pagesCase Alcohol Abuse and Unusual Abdominal Pain in A 49-Year-OldPutri AmeliaNo ratings yet
- 2 Haematuria & Bladder CancerDocument37 pages2 Haematuria & Bladder Cancerallthingali217No ratings yet
- Abses HeparDocument4 pagesAbses HeparRizki KhairNo ratings yet
- All About Pleural EffusionDocument6 pagesAll About Pleural EffusionTantin KristantoNo ratings yet
- Liver Abscess Causes, Symptoms and TreatmentDocument3 pagesLiver Abscess Causes, Symptoms and Treatmentras emil sazuraNo ratings yet
- Case Abdominal Pain in A 49-Year-OldDocument6 pagesCase Abdominal Pain in A 49-Year-OldPutri AmeliaNo ratings yet
- Abses Hati PiogenikDocument18 pagesAbses Hati Piogenikhasan andrianNo ratings yet
- Amoebic Liver Abscess: Clinical MedicineDocument5 pagesAmoebic Liver Abscess: Clinical MedicineAizat KamalNo ratings yet
- Parasitic Infections in ICU: Amoebiasis & SchistosomiasisDocument10 pagesParasitic Infections in ICU: Amoebiasis & SchistosomiasisPc MigasNo ratings yet
- Peritonitis and Abdominal SepsisDocument39 pagesPeritonitis and Abdominal Sepsisshelly_shellyNo ratings yet
- Liver Abcess & TumorsDocument73 pagesLiver Abcess & Tumorsasyraf asariNo ratings yet
- BMJ AscitesDocument30 pagesBMJ Ascitesaliakbar178No ratings yet
- Gallbladder and Bile Duct Anatomy, Function and DiseasesDocument16 pagesGallbladder and Bile Duct Anatomy, Function and DiseasesKadenceFreya-Charisse G PosadasBulintao100% (2)
- Ruptured hepatic abscess mimicking perforated viscusDocument3 pagesRuptured hepatic abscess mimicking perforated viscusMichu VuNo ratings yet
- Splenic Abscess: BackgroundDocument4 pagesSplenic Abscess: BackgroundRizki KhairNo ratings yet
- Cholangitis: Causes, Diagnosis, and ManagementDocument10 pagesCholangitis: Causes, Diagnosis, and ManagementDianaNo ratings yet
- Urinary Tract Infections (UTI) in Diabetes Mellitus - Overview, Renal Emphysema, Emphysematous CystitisDocument5 pagesUrinary Tract Infections (UTI) in Diabetes Mellitus - Overview, Renal Emphysema, Emphysematous CystitisDuy BiNo ratings yet
- Emergent Treatment of Acute Cholangitis andDocument10 pagesEmergent Treatment of Acute Cholangitis anddhimas satriaNo ratings yet
- Bowel Obstruction: A Narrative Review For All PhysiciansDocument7 pagesBowel Obstruction: A Narrative Review For All PhysiciansadeNo ratings yet
- Cholecystitis: Kaitlyn J. Kelly and Sharon Marie WeberDocument10 pagesCholecystitis: Kaitlyn J. Kelly and Sharon Marie WeberMasithaNo ratings yet
- Biliary Diseases: Dr. Wu Yang Dept. of Surgery The First Affiliated Hospital of Zhengzhou UniversityDocument75 pagesBiliary Diseases: Dr. Wu Yang Dept. of Surgery The First Affiliated Hospital of Zhengzhou Universityapi-19916399No ratings yet
- Cirrhosis of LiverDocument6 pagesCirrhosis of LiverpakdejackNo ratings yet
- Ascitis en Los Pacientes Oncológicos. Fisiopatogenia y Opciones de TratamientoDocument7 pagesAscitis en Los Pacientes Oncológicos. Fisiopatogenia y Opciones de TratamientoAngelica Figueroa MancillaNo ratings yet
- Acute Suppurative Cholangitis: Edward Chock, M.D., Bruce Wolfe, M.D., and Nathaniel Matolo, M.DDocument8 pagesAcute Suppurative Cholangitis: Edward Chock, M.D., Bruce Wolfe, M.D., and Nathaniel Matolo, M.DAhmad Harissul IbadNo ratings yet
- Differential Diagnosis of Pleural Effusions: Review ArticleDocument0 pagesDifferential Diagnosis of Pleural Effusions: Review ArticleRona SetiawatiNo ratings yet
- 260-Article Text-875-1-10-20220728Document4 pages260-Article Text-875-1-10-20220728hasan andrianNo ratings yet
- Choledocholithiasis - Clinical Manifestations, Diagnosis, and Management - UpToDateDocument30 pagesCholedocholithiasis - Clinical Manifestations, Diagnosis, and Management - UpToDateAdriana Barrios EscuderoNo ratings yet
- 15 Proximal Biliary MalignacyDocument20 pages15 Proximal Biliary MalignacyMars JambiNo ratings yet
- Complications of PancratitisDocument14 pagesComplications of PancratitisjulieNo ratings yet
- Pylephlebitis Incidentally Found On A Patient With Gastric CancerDocument3 pagesPylephlebitis Incidentally Found On A Patient With Gastric CancerTony NouhNo ratings yet
- CholangitisDocument4 pagesCholangitisNarianne Mae Solis Bedoy100% (1)
- Major Causes of Upper GI Bleeding in AdultsDocument22 pagesMajor Causes of Upper GI Bleeding in AdultsInna MaynizaNo ratings yet
- Cholestatic Liver Disease Guide for Diagnosis and ManagementDocument9 pagesCholestatic Liver Disease Guide for Diagnosis and ManagementDaniel Fernando LozanoNo ratings yet
- Liver Abscess DissertationDocument4 pagesLiver Abscess DissertationPayForAPaperAtlanta100% (1)
- Aghiz 3Document12 pagesAghiz 3nandaaa aprilNo ratings yet
- Causes of Upper Gastrointestinal Bleeding in Adults UpToDateDocument37 pagesCauses of Upper Gastrointestinal Bleeding in Adults UpToDateJodene Rose RojasNo ratings yet
- Absceso Hepatico HarrisonDocument2 pagesAbsceso Hepatico HarrisonZafiss ChaconNo ratings yet
- AIDS CholangiopathyDocument7 pagesAIDS CholangiopathyedwincliffordNo ratings yet
- A Case Study of Mesenteric Ischemia by Low Flow CT ImagingDocument5 pagesA Case Study of Mesenteric Ischemia by Low Flow CT ImagingAmanda SmithNo ratings yet
- Colelitiasis SD MirizziDocument14 pagesColelitiasis SD MirizziPipex Del MorroNo ratings yet
- Maat 12 I 3 P 271 DDDDDocument5 pagesMaat 12 I 3 P 271 DDDDKacamata BagusNo ratings yet
- Dr. S.P. Hewawasam (MD) Consultant Gastroenterologist/Senior Lecturer in PhysiologyDocument33 pagesDr. S.P. Hewawasam (MD) Consultant Gastroenterologist/Senior Lecturer in PhysiologyAjung SatriadiNo ratings yet
- Liver Abscess Background, Causes, Symptoms and ManagementDocument4 pagesLiver Abscess Background, Causes, Symptoms and ManagementLadywill GamitNo ratings yet
- Acute cholecystitis pathogenesis, diagnosis and clinical featuresDocument38 pagesAcute cholecystitis pathogenesis, diagnosis and clinical featuresAdrian BăloiNo ratings yet
- Acute Cholecystitis Diagnosis and TreatmentDocument16 pagesAcute Cholecystitis Diagnosis and TreatmentAndrés Menacho AbularachNo ratings yet
- FRCR Physics MCQs in Clinical Radiology Exam 1 ADocument16 pagesFRCR Physics MCQs in Clinical Radiology Exam 1 ADr. BongaNo ratings yet
- Quality Control of CTDocument6 pagesQuality Control of CTFrancisco HernandezNo ratings yet
- Cronicon: Review Article Approach To Acute Abdomen in The Primary Health Care SettingDocument6 pagesCronicon: Review Article Approach To Acute Abdomen in The Primary Health Care SettingtamiNo ratings yet
- Review Ct-GuidingDocument10 pagesReview Ct-GuidingHeru SigitNo ratings yet
- Evidence Based Management of Acute Musculoskeletal Pain 2Document259 pagesEvidence Based Management of Acute Musculoskeletal Pain 2api-26227671100% (4)
- Cardiology PDFDocument3 pagesCardiology PDFAamir HamaadNo ratings yet
- Iec 60601 2 44 2001 Amd1 2002 CSV en PDFDocument11 pagesIec 60601 2 44 2001 Amd1 2002 CSV en PDFvk9535No ratings yet
- CT Teaching Manual A Systematic Approach To CT Reading, 2nd EditionDocument304 pagesCT Teaching Manual A Systematic Approach To CT Reading, 2nd Editionvivin andcraftNo ratings yet
- CT BrochureDocument4 pagesCT BrochureRicardo KanayamaNo ratings yet
- Image Sensing and AcquisitionDocument5 pagesImage Sensing and AcquisitionThilagavathi75% (4)
- B90 Digital Bus Relay ProtectionDocument71 pagesB90 Digital Bus Relay ProtectionHamayoun MurtazaNo ratings yet
- Diagrams For X-Series MonitorDocument7 pagesDiagrams For X-Series MonitorRashid Kh100% (1)
- F02168en-00Document8 pagesF02168en-00JorgeCorkeNo ratings yet
- Targeted Percutaneous Microwave Ablation at The Pulmonary Lesion Combined With Mediastinal Radiotherapy With or Without Concurrent Chemotherapy inDocument8 pagesTargeted Percutaneous Microwave Ablation at The Pulmonary Lesion Combined With Mediastinal Radiotherapy With or Without Concurrent Chemotherapy inMalekseuofi مالك السيوفيNo ratings yet
- Differential ProtectionDocument30 pagesDifferential Protectiononderarslan77100% (4)
- SIBICCDocument12 pagesSIBICCnindyaNo ratings yet
- Cruz v. Felicisimo v. AgasDocument2 pagesCruz v. Felicisimo v. AgasKaren Joy MasapolNo ratings yet
- Bifid Mandibular Canal in Japanese: B M C JDocument9 pagesBifid Mandibular Canal in Japanese: B M C JBraily CardenasNo ratings yet
- Acute Pyelonephritis - Correlation of Clinical Parameter With Radiological Imaging AbnormalitiesDocument7 pagesAcute Pyelonephritis - Correlation of Clinical Parameter With Radiological Imaging AbnormalitiesKhristine Caye FernandezNo ratings yet
- Boardworks A2 Physics Contents Guide Boa PDFDocument21 pagesBoardworks A2 Physics Contents Guide Boa PDFshahbaz manzoorNo ratings yet
- Fundamentals of Bus Bar ProtectionDocument92 pagesFundamentals of Bus Bar ProtectionNorng VibolNo ratings yet
- 4 Oral Cavity ProceduresDocument10 pages4 Oral Cavity ProceduresAnne MarieNo ratings yet
- RCDSO Standard of Practice Dental CT ScannersDocument8 pagesRCDSO Standard of Practice Dental CT Scannersl4j0b9No ratings yet
- Powerlogic Energy Meter: Instruction Boletín de Bulletin Instrucciones EnglishDocument28 pagesPowerlogic Energy Meter: Instruction Boletín de Bulletin Instrucciones EnglishJose LunaNo ratings yet
- CV MTRDocument8 pagesCV MTRMijanur RahmanNo ratings yet