Professional Documents
Culture Documents
Quantum Mechanics:Uncertainty Principle
Uploaded by
vivek patelCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Quantum Mechanics:Uncertainty Principle
Uploaded by
vivek patelCopyright:
Available Formats
Lecture 4
Uncertainty Principle
References :
1.Concept of Modern Physics
by Arthur Beiser
2. Modern Physics
by Kenneth Krane
2
4
3
A localized wave or wave packet:
Spread in position Spread in momentum
Superposition of waves
of different wavelengths
to make a packet
Narrower the packet , more the spread in momentum
Basis of Uncertainty Principle
A moving particle in quantum theory
Heisenberg's Uncertainty Principle
___________________________________
- The Uncertainty Principle is an important
consequence of the wave-particle duality of
matter and radiation and is inherent to the
quantum description of nature
- Simply stated, it is impossible to know both the
exact position and the exact momentum of an
object simultaneously
A fact of Nature!
Heisenberg's Uncertainty Principle
__________________________________
Uncertainty in Position :
Uncertainty in Momentum:
x A
x
p A
t 2
h
p x
x
> A A
Heisenberg's Uncertainty Principle
- applies to all conjugate variables
___________________________________
Position & momentum
Energy & time
t 2
h
p x
x
> A A
t 2
h
t E > A A
Uncertainty Principle and the Wave Packet
___________________________________
p
h
=
t 2
h
p x
x
> A A
p
p A
=
A
x A
Some consequences of the Uncertainty Principle
___________________________________
The path of a particle (trajectory) is not well-defined in
quantum mechanics
- Electrons cannot exist inside a nucleus
- Atomic oscillators possess a certain amount of energy
known as the zero-point energy, even at absolute zero.
Why is nt the uncertainty principle apparent to
us in our ordinary experience?
Plancks constant, again!!
___________________________________
Plancks constant is so small that the
uncertainties implied by the principle are also
too small to be observed. They are only
significant in the domain of microscopic
systems
J.s 10 x 6 . 6
34
= h
t 2
h
p x
x
> A A
Heisenberg Uncertainty Principle
The uncertainty principle states that the position
and momentum cannot both be measured,
exactly, at the same time.
Where h (6.6 x 10
-34
) is called Plancks constant. As h is so small, these
uncertainties are not observable in normal everyday situations
Ax Ap > h
The more accurately you
know the position (i.e., the
smaller Ax is) , the less
accurately you know the
momentum (i.e., the larger
Ap is); and vice versa
h or
2
h
or
t
For
Numerical
For
Applications
Historic importance
Increasing levels of wavepacket localization, meaning the
particle has a more localized position.
In the limit 0, the particle's
position and momentum become known
exactly. This is equivalent to the
classical particle.
p is less
p is more
12
The wave nature to particles means a particle is a wave packet,
the composite of many waves
Many waves = many momentums, observation makes one
momentum out of many.
Principle of complementarity: The moving electron will
behave as a particle or as a wave, but we can not observe both
aspects of its behavior simultaneously. It states that
complete description of a physical entity such as a
photon or an electron can not be made in terms of
only particle properties or only wave properties, but
that both aspects of its behavior must be considered.
Exact knowledge of complementarities pairs (position, energy,
time) is impossible.
Heisenberg Uncertainty Principle
Same situation, but baseball replaced by an electron which has mass 9.11
x 10
-31
kg
So momentum= 3.6 x 10
-29
kg m/s and Ap = 3.6 x 10
-31
kg m/s
The uncertainty in position is then
Example
A pitcher throws a 0.1-kg baseball at 40 m/s
So momentum is 0.1 x 40 = 4 kg m/s
Suppose the momentum is measured to an accuracy of 1 % , i.e.,
Ap = 0.01
p = 4 x 10
-2
kg m/s
The uncertainty in position is then
No wonder one does not observe the effects of the uncertainty principle in
everyday life!
Example: A free 10eV electron moves in the x-direction with a speed of
1.8810
6
m/s. assume that you can measure this speed to precision of 1%.
With what precision can you simultaneously measure its position?
the momentum of e- is
p
x
= m v
x
= 9.1110
-31
kg 1.8810
6
m/s
= 1.71 10
-24
kg m/s
The uncertainty A p
x
in momentum is 1%
Ax > h/4t A p
x
=3.1 n m
Example: A golf ball has a mss 45gm and speed of 40 m/s, which you can
measure with a precision of 1%. What limits does the uncertainty principle
place on your ability to measure its position.
Calculations yields Ax > 6 10
-31
m. this is very small distance,
14
15
Applications of uncertainty principle
1. Non-existence of electrons in the Nucleus
Assume that the electron is present in the nucleus. The radius of
the nucleus of any atom is of the order 5 fermi (1 fermi = 10
-15
m). For
the existence of electron in the nucleus, the uncertainty Ax in its
position would be at least equal to the radius of the nucleus, i.e.
uncertainty in the position
According to the uncertainty principle.
Ap > h/4tAx= 1.05410
-20
kg-m/sec
If this is the uncertainty in momentum of the electron then the
momentum of the electron must be at least of the order of its
magnitude, that is , p ~ 1.05410
-20
kg-m/sec, an electron having so
much momentum should have a velocity comparable to the velocity
of light. Hence, its energy should be calculated by the relativistic
formula
E
2
=p
2
c
2
+m
o
2
c
4
15
x R 5 10 m
A = =
16
pc= 1.05410
-20
310
8
= 20 MeV
The rest energy of electron 0.51 MeV, is very small as compared to
pc. Hence second term in relativistic equation can be neglected.
Thus, if the electron is the constituents of the nucleus, it should
have an energy of the order of 20 MeV.
However, from experiment of decay it is found that the
electrons emitted from the radioactive element do not have more
than 2-3 MeV. Therefore, it is confirmed that electrons do not
reside inside the nucleus.
Applications of uncertainty principle
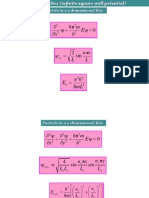
4. Particle in a Box Problem
Find the minimum energy for a particle confined to a box of size L
Macroscopic: 1 g particle confined to 1 m E
min
~ 10
-29
eV
Microscopic: Electron confined to 0.1 nm E
min
~ 4 eV
2 2
2 2
min
34 16
2
min
2
Using (Uncertainty Principle) and
where
(for 0),
2 2
1.05 10 J s or 6.58 10 eV s
2
p p p p
x
p p
E
m m
E
mL
A = A = =
A
A
= =
=
=
x = L
x
X=0
X=L
Energy
Applications of uncertainty principle
Physical Origin of the Uncertainty Principle
Heisenberg (Bohr) Microscope
The measurement itself introduces
the uncertainty
When we look at an object we see it
via the photons that are detected by
the microscope
These are the photons that are scattered
within an angle 2 and collected by a
lens of diameter D
Momentum of electron is changed
Consider single photon, this will
introduce the minimum uncertainty
u sin 2
max
ph ph
p p = A
u sin 2
ph ph electron
p p p = A = A
u
u u u
u
h
p
h
p p
h
p
electron
ph electron
ph
2
sin , small for
sin
2
sin 2
= A
~
= = A
=
As a consequence of
momentum conservation
Physical Origin of the Uncertainty Principle
Heisenberg (Bohr) Microscope
Trying to locate electron we
introduce the uncertainty of
the momentum
~(D/2)/L, L ~ D/2 is
distance to lens
Uncertainty in electron
position for small is
To reduce uncertainty in the
momentum, we can either
increase the wavelength or
reduce the angle
But this leads to increased
uncertainty in the position,
since
u h
p
electron
2
= A
electron
x
2sin 2
A = =
u u
Physical Origin of the Uncertainty Principle
Heisenberg (Bohr) Microscope
electron
electron
electron
electron
2h
p
h
p
x
2 x
( p )( x) h
( p )( x)
A = u
A =
`
A
u =
A )
A A =
A A >
Physical Origin of the Uncertainty Principle
Heisenberg (Bohr) Microscope
26
To see more clearly into the nature of uncertainty, we consider
electrons passing through a slit:
We apply the condition of
minima from single slit
diffraction,
and postulate that is the de
Broglie wavelength.
Momentum
uncertainty in the
y component
P
x
=h/
Experimental illustration of Uncertainty Principle: Single slit
diffraction.
y y
x
y
y
sin =
for small sin tan
p p
tan
p h /
p
p h
h /
u
e
u u ~ u
A A
u = =
A
= A e=
e
Since the electron can pass the slit
through anywhere over the width ,
the uncertainty in the y position of the
electron is Ay=.
p y h A A ~
sin n e u =
27
which is in agreement with the uncertainty principle. If we try to
improve the accuracy of the position by decreasing the width of the slit,
the diffraction pattern will be widened. This means that the
uncertainty in momentum will increase.
The uncertainty principle is applicable to all material particles,
from electrons to large bodies occurring in mechanics. In case of large
bodies, however , the uncertainties are negligibly small compared to
the ordinary experimental errors.
y
p y h A A ~
28
2
2
and : packet ave Gaussian w a For
> A A > A A t E x p
. 1 then , and Given ~ A A A = A ~ A A x p k p x k
. 1 then , and Given ~ A A A = A ~ A A t E E t e e
Particle is highly localized in space only if its momentum is undefined.
Particles energy is accurate only if measured for a long time.
Heisenberg Uncertainty Principle
Example: Assume the position of an object is known so
precisely that the uncertainty in the position is only
Ay=1.510
-11
m. Determine the minimum uncertainty in
the momentum of the object and find the corresponding
minimum uncertainty in the speed if the object in an
electron.
Ap
y
=h/(4tAy)=(6.6310
-34
Js)/(4t 1.510
-11
m)
Ap
y
=3.510
-24
kg m/s small
Av
y
=Ap
y
/m=(3.510
-24
kg m/s)/(9.110
-31
kg)
Av
y
=3.910
6
m/s large
29
You might also like
- Mod Phy e by M Experiment e by M DoneDocument8 pagesMod Phy e by M Experiment e by M Doneapi-3723453No ratings yet
- Unit 7 PhaseruleDocument11 pagesUnit 7 Phaseruleengineeringchemistry100% (2)
- Dispersion and Alignment of Carbon Nanotubes in Polymer Matrix A ReviewDocument24 pagesDispersion and Alignment of Carbon Nanotubes in Polymer Matrix A ReviewYovaraj Karunakaran0% (1)
- Development and Characterization of Mixed Halide Perovskite Solar CellsDocument19 pagesDevelopment and Characterization of Mixed Halide Perovskite Solar CellsSpandan AnupamNo ratings yet
- CrystallographyDocument95 pagesCrystallographyAswith R ShenoyNo ratings yet
- Practical Booklet Paper 2Document22 pagesPractical Booklet Paper 2Cutry CarryNo ratings yet
- Composite MaterialsDocument30 pagesComposite Materialsarvindvsk23No ratings yet
- Nanotechnology - Academic Essay Assignment - WWW - TopgradepapersDocument14 pagesNanotechnology - Academic Essay Assignment - WWW - TopgradepapersTop Grade Papers100% (1)
- Experiment: 4.3 Understanding TransistorDocument1 pageExperiment: 4.3 Understanding TransistorFaris ShahinNo ratings yet
- Introduction To Nanotechnology and Its Applications in Forensic Investigation and AnalysisDocument5 pagesIntroduction To Nanotechnology and Its Applications in Forensic Investigation and AnalysisEditor IJTSRD100% (1)
- Carbon Nanotube CompositesDocument8 pagesCarbon Nanotube CompositesgnanalakshmiNo ratings yet
- Perovskite Solar Cell NOTESDocument3 pagesPerovskite Solar Cell NOTESSudhir RanjanNo ratings yet
- Biodegradable Polymer Matrix Nanocomposites For Tissue Engineering PDFDocument21 pagesBiodegradable Polymer Matrix Nanocomposites For Tissue Engineering PDFJuan Pablo BulaciosNo ratings yet
- The Structure and Properties of Polymers: Also Known AsDocument15 pagesThe Structure and Properties of Polymers: Also Known AsDiki yunikaNo ratings yet
- The Moral Black HoleDocument3 pagesThe Moral Black HoleJoydeep NaskarNo ratings yet
- SynopsisDocument3 pagesSynopsisNaresh Lalwani100% (1)
- Nanocomposites Used In: Food Packaging IndustryDocument8 pagesNanocomposites Used In: Food Packaging IndustryPrashant VermaNo ratings yet
- 4.2 DiodeDocument1 page4.2 DiodeFaris ShahinNo ratings yet
- Quantum Mechanics Tunneling & Harmonic OscillatorDocument33 pagesQuantum Mechanics Tunneling & Harmonic Oscillatorvivek patelNo ratings yet
- BSC Physics PDFDocument138 pagesBSC Physics PDFAbhishikth Joseph Anand100% (1)
- 5.1 Nucleus of AtomDocument1 page5.1 Nucleus of AtomFaris ShahinNo ratings yet
- Maluss LawDocument2 pagesMaluss LawJoydeep NaskarNo ratings yet
- Modern Physics 4Document1 pageModern Physics 4aditya shresthaNo ratings yet
- Unit 3 PolymersDocument19 pagesUnit 3 PolymersengineeringchemistryNo ratings yet
- Carbon Nanotube Reinforced Composites Potential and Current ChallengesDocument8 pagesCarbon Nanotube Reinforced Composites Potential and Current ChallengesMuhammad AdnanNo ratings yet
- Nanotechnology Lesson For MS StudentsDocument6 pagesNanotechnology Lesson For MS StudentssavithatssNo ratings yet
- Lecture-7: Electric Potential, Laplace and Poisson's EquationsDocument38 pagesLecture-7: Electric Potential, Laplace and Poisson's Equationsvivek patelNo ratings yet
- 10 Dual Nature of Matter and RadiationDocument7 pages10 Dual Nature of Matter and RadiationnagarajanNo ratings yet
- 9 Focused Ion Beam MicrosDocument28 pages9 Focused Ion Beam MicrosPrashant VermaNo ratings yet
- CHAPTER 6-Jfet Part ADocument51 pagesCHAPTER 6-Jfet Part Adeafrida oxaura100% (2)
- Organic and Perovskite Solar Cells Working Principles, Materials and Interfaces - Marinova - 2016Document17 pagesOrganic and Perovskite Solar Cells Working Principles, Materials and Interfaces - Marinova - 2016Aniello LangellaNo ratings yet
- Electronic MeasurementsDocument66 pagesElectronic MeasurementsBenson Mansingh P MNo ratings yet
- Bloch TheoremDocument2 pagesBloch TheoremSurajNo ratings yet
- EMT Lecture Notes TECDocument139 pagesEMT Lecture Notes TECVishwajit SonawaneNo ratings yet
- Mission Jam 2023: Shanu AroraDocument11 pagesMission Jam 2023: Shanu AroraJatin PalNo ratings yet
- Central+Force 1Document43 pagesCentral+Force 1aljawharahksaNo ratings yet
- M.Tech Nanotechnology Syllabus in PSGDocument11 pagesM.Tech Nanotechnology Syllabus in PSGumapathi_nanoNo ratings yet
- CV ShobanaDocument4 pagesCV ShobanashobanaNo ratings yet
- Transducers and Data Acquisition SystemsDocument176 pagesTransducers and Data Acquisition SystemsBenson Mansingh P MNo ratings yet
- Failure of Classical MechanicsDocument25 pagesFailure of Classical MechanicsRasikh JalalNo ratings yet
- Unit 2 NotesDocument10 pagesUnit 2 NotesG.MUNEER BABA,EEE18 Vel Tech, ChennaiNo ratings yet
- Electron and PhotonDocument20 pagesElectron and Photonnitin100% (2)
- Band Theory of Solids: Brajesh Tiwari and R. E. AmritkarDocument9 pagesBand Theory of Solids: Brajesh Tiwari and R. E. AmritkarkapilNo ratings yet
- Interatomic Forces: Solid State Physics by S.O.Pillai)Document17 pagesInteratomic Forces: Solid State Physics by S.O.Pillai)vivek patelNo ratings yet
- Unit 6 Class Notes MEMs & NanotechnologyDocument25 pagesUnit 6 Class Notes MEMs & NanotechnologynitinNo ratings yet
- Chapter 5: Surface Area To Volume Ratios in Nanoscience: Table ofDocument3 pagesChapter 5: Surface Area To Volume Ratios in Nanoscience: Table ofamanNo ratings yet
- $electrostaticsDocument24 pages$electrostaticsAtul VermaNo ratings yet
- Intro To SemiconductorsDocument27 pagesIntro To SemiconductorsRolando CelesteNo ratings yet
- Particle in A 1d Box Quantum MechanicsDocument22 pagesParticle in A 1d Box Quantum Mechanicsvivek patel0% (1)
- 8-Ch7 (Struktur Atom)Document91 pages8-Ch7 (Struktur Atom)Mia YukimuraNo ratings yet
- Optical Fibers: Structures, Optical Fibers: Structures, Waveguiding & FabricationDocument99 pagesOptical Fibers: Structures, Optical Fibers: Structures, Waveguiding & FabricationNung NingNo ratings yet
- Central ForceDocument59 pagesCentral Forcenuriwulandari100% (1)
- Nanotechnology Syllabus Jntu KakinadaDocument2 pagesNanotechnology Syllabus Jntu Kakinadasagar100% (2)
- PolymersDocument140 pagesPolymersNitin Nishant100% (2)
- Alternating CurrentDocument39 pagesAlternating CurrentRichard GomezNo ratings yet
- SuperconductorDocument33 pagesSuperconductorCrisanta GanadoNo ratings yet
- EN292 MaterialsDocument80 pagesEN292 MaterialsGrant HeilemanNo ratings yet
- The Heisenberg Uncertainty Principle Final 2014Document58 pagesThe Heisenberg Uncertainty Principle Final 2014Asad ChoudaryNo ratings yet
- Heisenberg Principle Uncertainity PDFDocument20 pagesHeisenberg Principle Uncertainity PDFRajanikanta SahuNo ratings yet
- Phy1 4Document5 pagesPhy1 4sakshamsharma7257No ratings yet
- Quadratic FormDocument3 pagesQuadratic Formvivek patelNo ratings yet
- Norm Condition NoDocument6 pagesNorm Condition Novivek patelNo ratings yet
- Vector Spaces: C Michael C. Sullivan, Fall 2005Document5 pagesVector Spaces: C Michael C. Sullivan, Fall 2005vivek patelNo ratings yet
- MatrixDocument9 pagesMatrixvivek patelNo ratings yet
- Matrices Solved ProblemsDocument19 pagesMatrices Solved Problemsvivek patel100% (1)
- Diffeq 3 Systems of Linear DiffeqDocument10 pagesDiffeq 3 Systems of Linear Diffeqvivek patelNo ratings yet
- Numerical Analysis Lecture Notes: 7. Iterative Methods For Linear SystemsDocument28 pagesNumerical Analysis Lecture Notes: 7. Iterative Methods For Linear Systemsvivek patel100% (1)
- Bottom Up Parsing1Document69 pagesBottom Up Parsing1vivek patelNo ratings yet
- Bottom Up Parsing-Shift Reduce ParsingDocument43 pagesBottom Up Parsing-Shift Reduce Parsingvivek patelNo ratings yet
- Linear FunctionalDocument5 pagesLinear Functionalvivek patelNo ratings yet
- Vector Spaces: Persson@berkeley - EduDocument4 pagesVector Spaces: Persson@berkeley - Eduvivek patelNo ratings yet
- Bottom Up ParsingDocument149 pagesBottom Up Parsingvivek patelNo ratings yet
- Date: 14/08/2014: Notice - I III Year CSE Minor Project-IDocument1 pageDate: 14/08/2014: Notice - I III Year CSE Minor Project-Ivivek patelNo ratings yet
- MOOD MetricDocument10 pagesMOOD Metricvivek patelNo ratings yet
- Mips Instruction FormatDocument41 pagesMips Instruction Formatvivek patelNo ratings yet
- Object Oriented MetricsDocument1 pageObject Oriented Metricsvivek patelNo ratings yet
- Cocomo ModelDocument17 pagesCocomo Modelvivek patelNo ratings yet
- Software EngineeringDocument2 pagesSoftware Engineeringvivek patelNo ratings yet
- Operating SystemDocument58 pagesOperating Systemvivek patelNo ratings yet
- Notice - Ii III Year CSE Minor Project-IDocument1 pageNotice - Ii III Year CSE Minor Project-Ivivek patelNo ratings yet
- Write 8086 Assemble Language Program To Print The Following Pattern at The Mid of The ScreenDocument1 pageWrite 8086 Assemble Language Program To Print The Following Pattern at The Mid of The Screenvivek patelNo ratings yet
- THESISDocument44 pagesTHESISRowena Shaira AbellarNo ratings yet
- Iso 657 14 2000 en FR PDFDocument11 pagesIso 657 14 2000 en FR PDFVivekanandh00333 VivekNo ratings yet
- Lesson Plan 3Document3 pagesLesson Plan 3api-547884261No ratings yet
- Routes of Medication AdministrationDocument2 pagesRoutes of Medication AdministrationTracy100% (6)
- Bombardier Zefiro Technical Description enDocument15 pagesBombardier Zefiro Technical Description ennickerlesstezla100% (1)
- Problem Set Solution QT I I 17 DecDocument22 pagesProblem Set Solution QT I I 17 DecPradnya Nikam bj21158No ratings yet
- A World of Composite Technologies BrochureDocument15 pagesA World of Composite Technologies Brochurethorsten_kasterNo ratings yet
- Basic Mechanical Engineering Btkit Dwarahat: Attempt All Questions. Q. 1. Attempt Any Four Parts: 5 X 4 20Document2 pagesBasic Mechanical Engineering Btkit Dwarahat: Attempt All Questions. Q. 1. Attempt Any Four Parts: 5 X 4 20anadinath sharmaNo ratings yet
- Scripture Passages Suitable For Lectio Divina: 1 John 4:7-11Document2 pagesScripture Passages Suitable For Lectio Divina: 1 John 4:7-11Victor AugustoNo ratings yet
- Chemical Tanker Familiarization (CTF) : Companies Can Opt For Block BookingDocument1 pageChemical Tanker Familiarization (CTF) : Companies Can Opt For Block BookingSamiulNo ratings yet
- NSC Solution F2 enDocument8 pagesNSC Solution F2 ensaeidNo ratings yet
- 8484.sensor CEM Diagnostic Tests User Manual v3.1.0Document28 pages8484.sensor CEM Diagnostic Tests User Manual v3.1.0Edgar FuentesNo ratings yet
- Comparative Study of Conventional and Generative Design ProcessDocument11 pagesComparative Study of Conventional and Generative Design ProcessIJRASETPublicationsNo ratings yet
- Poster - Combur10 Test Parameters PDFDocument1 pagePoster - Combur10 Test Parameters PDFAde FeriyatnaNo ratings yet
- Chapter 8 - Nervous ReviewerDocument18 pagesChapter 8 - Nervous Reviewerchristian anchetaNo ratings yet
- Theories of DissolutionDocument17 pagesTheories of DissolutionsubhamNo ratings yet
- Hostel B Menu From 16 March To 31 March'2024Document4 pagesHostel B Menu From 16 March To 31 March'2024govindkauNo ratings yet
- 03-CircO2 Previous Control Nitric OxideDocument17 pages03-CircO2 Previous Control Nitric OxideVic SpeaksNo ratings yet
- Object: Annex A, B, C DDocument74 pagesObject: Annex A, B, C DfjsdNo ratings yet
- STAT 713 Mathematical Statistics Ii: Lecture NotesDocument152 pagesSTAT 713 Mathematical Statistics Ii: Lecture NotesLiban Ali MohamudNo ratings yet
- PPG 2020Document131 pagesPPG 2020Syed Rohail AhmedNo ratings yet
- 002-679e-08.19 v1.6.x KLDocument523 pages002-679e-08.19 v1.6.x KLChanon OnramoonNo ratings yet
- Hemiplegia LectureDocument37 pagesHemiplegia LectureRancesh FamoNo ratings yet
- Craig - 4353 TX CobraDocument3 pagesCraig - 4353 TX CobraJorge ContrerasNo ratings yet
- TDC Calculation For The Determination of Drill Bit PerformanceDocument3 pagesTDC Calculation For The Determination of Drill Bit Performancejanuar baharuliNo ratings yet
- Fiber Testing and OTDR Basics: Brett Isley Terriitory Sales ManagerDocument54 pagesFiber Testing and OTDR Basics: Brett Isley Terriitory Sales ManagerTuppiNo ratings yet
- BBMP C&D Draft Notice 2014 (Updated)Document10 pagesBBMP C&D Draft Notice 2014 (Updated)PriankMathurNo ratings yet
- Coffee Vibes: Here Is Where Your Presentation BeginsDocument86 pagesCoffee Vibes: Here Is Where Your Presentation Beginssyeda salmaNo ratings yet
- 12 Elements of Firearms TrainingDocument6 pages12 Elements of Firearms TraininglildigitNo ratings yet
- Introducing RS: A New 3D Program For Geotechnical AnalysisDocument4 pagesIntroducing RS: A New 3D Program For Geotechnical AnalysisAriel BustamanteNo ratings yet