Professional Documents
Culture Documents
2012-Fluid and Electrolytes
Uploaded by
kyuss20 ratings0% found this document useful (0 votes)
50 views192 pagesfe
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentfe
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
50 views192 pages2012-Fluid and Electrolytes
Uploaded by
kyuss2fe
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 192
HARLEY L.
DELA CRUZ RN MAN
Instructor
Phospholipid bilayer
Freely permeable to non-polar
molecules (CO
2
, O
2
, steroids)
Impermeable to large polar and
charged molecules (ions,
proteins, glucose)
Generally permeable to water (though some
cells require aquaporins)
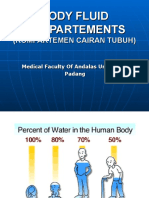
Approx water
content in body
Age group
90% Premature infant
70-80% Newborn infant
64% 12 - 24 months
60% Adult
60%
fluids
55%
fluids
Total Body Mass
female male
45%
solids
40%
solids
2/3
Intra-
cellular
fluid
(ICF)
1/3
(ECF)
80%
20%
Interstitial
fluid
Plasma
Some fluid is lost from blood in the
interstitial tissues, and returned by the
lymphatic system
(also lymph and other
miscellaneous fluids)
5
2/3 (65%) of TBW is intracellular (ICF)
1/3 extracellular water
25 % interstitial fluid (ISF)
5- 8 % in plasma (IVF intravascular fluid)
1- 2 % in transcellular fluids CSF, intraocular
fluids, serous membranes, and in GI, respiratory
and urinary tracts (third space)
Function of ICF & ECF:
ICF: is vital to normal cell function, its contain
solutes such as oxygen, electrolytes and
glucose. It provides a medium to metabolic
process.
ECF: it is the transport system that carries
nutrients and waste product from the cell.
The proportion of water decreases with aging because fat,
age and sex effect of total body water.
Infants have a greater proportion of extracellular fluid
than older children and adults.
Because extracellular fluid is more easily lost from
the body than intracellular fluid, infants are more at
risk of developing dehydration than older children and
adults (infants also have a larger surface area to body
mass ratio).
Na
+
Cl
-
HCO
3
-
K
+
Mg
2+
PO
4
3-
+
+
+
+
-
-
-
-
9
Electrolytes charged particles
Cations positively charged ions
Na
+
, K
+
, Ca
++
, H
+
Anions negatively charged ions
Cl
-
, HCO
3
-
, PO
4
3-
Non-electrolytes - Uncharged
Proteins, urea, glucose, O
2
, CO
2
ICF (mEq/L) ECF (mEq/L)
Sodium 20 135-145
Potassium 150 3-5
Chloride --- 98-110
Bicarbonate 10 20-25
Phosphate 110-115 5
Protein 75 10
o Osmolarity = solute/(solute+solvent)
o Osmolality = solute/solvent (275-295 mOsm/L)
o Tonicity = effective osmolality
o Plasma osmolility = 2 x (Na) + (Glucose/18) +
(Urea/2.8)
o Plasma tonicity = 2 x (Na) + (Glucose/18)
MW (Molecular Weight) = sum of the weights of
atoms in a molecule
mEq (milliequivalents) = MW (in mg)/ valence
mOsm (milliosmoles) = number of particles in a
solution
13
Tonicity
Isotonic
Hypertonic
Hypotonic
14
15
Cell in a
hypotonic
solution
16
Cell in a
hypertonic
solution
Diffusion
Osmosis
Filtration
Active transport
1. Osmosis:
Is the movement of water across cell
membranes, from the less concentrated
solution to more concentrated solution. In other
word water move toward higher concentration.
Solutes are substance dissolved in liquid.
Crystalloid: salts that dissolved readily in to true solution.
Colloids: substance such as large protein molecules that
do not dissolved in true solution.
Sodium is the major determinant of serum
osmolality.
2. Diffusion:
Is the continual intermingling of molecules in
liquid, gases by random movement of the
molecules.
3. Filtration:
Is the process where by fluid and solutes moved
together across a membrane from one compartment to
another.
4. Active transport:
substance can move across cell membranes
from a less concentrated solution to amore
concentrated one by active transport.
Sodium and potassium concentrations in extra- and
intracellular fluids are nearly opposite
This reflects the activity of ATP-dependent sodium-potassium
pumps (Na
+
-K
+
ATPase)
Continuous exchange and mixing of fluid among
compartments - regulated by osmotic and hydrostatic
pressures
Net leakage of fluid from the blood is picked up by lymphatic
vessels and returned to the bloodstream
Exchanges between interstitial and intracellular fluids are
more complex due to the selective permeability of the cell
membranes
An increase in ECF solute concentration [NaCl] would cause osmotic and
volume changes in the ICF.
Which way would water move, into or out of cells?
ICF is determined by the ECF solute concentration
solute
solute
solute
solute
solute
solute
solute
solute
solute
solute
More Solute = Less Water Less Solute = More Water
Hypertonic Solution or
Hypotonic Solution?
solute
solute
solute
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Which way will Water move?
solute
solute
solute
solute
solute
solute
solute
solute
solute
solute
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
If the oncotic pressure in the interstitium increased, would this promote or
inhibit the re-entry of fluid in a capillary bed?
H
2
O
Daily water intake must equal water output
Water Intake Water Output
Stimulated by thirst
center of
hypothalamus
Osmoreceptors
detect an increase
in fluid osmolarity
Thirst center inhibited by
distension of stomach wall
Sensible loss: urine,
feces, noticible sweat
Insensible loss:
respiration and non-
noticible sweat
Urine output is the primary regulator of
water out (ADH from posterior pituitary
gland)
Water intake:
Ingested fluid (60%) and solid food (30%)
Metabolic water or water of oxidation (10%)
Water output:
Urine (60%) and feces (4%)
Lost via lungs and skin (28%), sweat (8%)
To remain properly hydrated, water intake must equal water output
Fluid Gain and Loss
Why are you told to drink plenty of fluids when you have a fever?
A fever increases water loss (maybe both insensible and sensible)
The hypothalamic thirst center is stimulated by:
A decline in plasma volume of 10%15%
Increases in plasma osmolality of 12%
Baroreceptor input, angiotensin II, etc.
Feedback signals that inhibit the thirst centers include:
Moistening of the mucosa of the mouth and throat
Activation of stomach and intestinal stretch receptors
Body fluids are:
Electrically neutral
Osmotically maintained
Specific number of particles per
volume of fluid
Ion transport
Water movement
Kidney function
Water loss (output) exceeds water intake and the
body is in negative fluid balance
A common sequala to hemorrhage, severe burns,
prolonged vomiting or diarrhea, profuse sweating,
water deprivation, and diuretic abuse
Signs and symptoms: dry mouth, thirst, dry flushed
skin, and oliguria
Prolonged dehydration may lead to weight loss,
fever, and mental confusion
Other consequences include hypovolemic shock and
loss of electrolytes
Accumulation of fluid in the interstitial space, leading to
tissue swelling, caused by anything that increases fluid
flow out of the bloodstream or hinders its return
Factors that accelerate fluid loss include:
Hypertension, increased capillary permeability, incompetent venous
valves, localized blood vessel blockage, congestive heart failure
Decreased fluid return usually reflects an imbalance in colloid
osmotic pressures across capillary membranes
Hypoproteinemia low levels of plasma proteins, may result from protein
malnutrition, liver disease, or glomerulonephritis
Fluids are forced out of capillary beds at the arterial ends by blood pressure, but
fail to return at the venous ends and interstitium becomes congested with fluid
Blocked (or surgically removed) lymph vessels may result
in the accumulation of plasma proteins in interstitial fluid
Interstitial colloid osmotic pressure increases,
fluid leaves blood and moves into tissue
Interstitial fluid accumulation could result in a
decrease in blood volume, blood pressure, and
impaired circulation
Kwashiorkor - a form of malnutrition caused by inadequate protein
intake and consequent reduced albumin in the blood
hypoalbuminemia and reduced plasma oncotic pressure promote the
extravasation of fluid from the plasma into the peritoneal cavity
Hypertension
Polyuria
Peripheral edema
Wet lung
Jugular vein engorgement
Especially when hypo-
albuminemia
Diminished skin turgor
Dry oral mucus membrane
Oliguria
- <500ml/day
- normal: 0.5~1ml/kg/h
Tachycardia
Hypotension
Hypoperfusioncyanosis
Altered mental status
Thorough history taking: poor intake, GI
bleedingetc
BUN : Creatinine > 20 : 1
- BUN: hyperalimentation, glucocorticoid
therapy, UGI bleeding
Increased specific gravity
Increased hematocrit
Electrolytes imbalance
Acid-base disorder
CVP
Pulse
Peripheral Veins
Weight
Thirst
Intake and Output
Skin
Edema
Lab Values
Preserve oxygen delivery to tissues
Correct hypovolaemia
Maintain cardiac output
Optimise gas exchange
Replace electrolytes & water
Maintain urine output
Colloids + RBCs
Crystalloids
Identify what is the goal
Choose fluid which best achieves the goal
Methods of estimating basal or maintenance
fluid requirements
Basal Surface Area
Need to know height and weight, requires table, does not allow
for deviations from normal activity
Basal or Calorie Expenditure Method
Requires a table, involves calculations, permits correction for
changes in activity or injury, drier
Holliday-Segar System
Easy to remember, does not require table or difficult
calculations, does not allow for deviations from normal activity
Isotonic crystalloids
- Lactated Ringers, 0.9% NaCl
- only 25% remain intravascularly
Hypertonic saline solutions
- 3% NaCl
Hypotonic solutions
- D5W, 0.45% NaCl
- less than 10% remain intra-
vascularly, inadequate for fluid
resuscitation
Contain high molecular weight
substancesdo not readily migrate across
capillary walls
Preparations
- Albumin: 5%, 25%
- Dextran
- Gelifundol
- Haes-steril 10%
Solutions Volumes Na
+
K
+
Ca
2+
Mg
2+
Cl
-
HCO
3
-
Dextrose mOsm/L
ECF 142 4 5 103 27 280-310
Lactated
Ringers
130 4 3 109 28 273
0.9% NaCl 154 154 308
0.45% NaCl 77 77 154
D5W
D5/0.45%
NaCl
77 77 50 406
3% NaCl 513 513 1026
6%
Hetastarch
500 154 154 310
5% Albumin 250,500
130-
160
<2.5
130-
160
330
25%
Albumin
20,50,100
130-
160
<2.5
130-
160
330
Common parenteral fluid therapy
0.9% Normal Saline
D5W 5 % Dextrose*
D51/4NS 5% Dextrose 0.2% NS
D51/3NS 5% Dextrose 0.3% NS
LR or RL Lactated Ringers Solution
3% N S 3% Normal saline
5 % N S 5% Normal Saline
D 10 W Dextrose 10% in water
D 20 W Dextrose 20% in Water
D5 NS 5%Dextrose,with 0.45% Normal Saline
D5NS 5% Dextrose with 0.9% Normal Saline
D5LR 5% Dextrose with Lactated Ringers
1/3 N S 0.33% Normal Saline
1/2 N S 0.45% Normal Saline
D 2.5 W Dextrose 2.5% in water
Neonates need relatively more fluid intake than older
infants and children.
The kidneys in neonates have small immature
glomeruli and for this reason the glomerular filtration
rate is reduced (about 30ml/min/1.73m2 at birth to
100ml/min/1.73m2 at nine months).
The loops of Henle are short and the distal
convoluted tubules are relatively resistant to
aldosterone, leading to a limited concentrating ability
For oral feeding with standard formula milk,
preterm babies may need 200ml/kg per day
initially.
Term babies need approximately 150ml/kg per
day until fully weaned.
Children and adolescents may drink up to 2-3
litres of fluid per day.
Hourly maintenance fluid requirements can be
calculated using the following guide:
4ml/kg/hr or 100ml/kg/day for first 10kg body Weight
2ml/kg/hr or 50ml/kg/day for second 10kg body Weight
1ml/kg/hr or 20ml/kg/day for each additional kg body
weight
The recommended volume of oral feeds is greater than
that calculated using this guide so that adequate
calorie and protein intake can be achieved.
ElECTROLYTE REQUIREMENTS
Na
+
3 mEq/100ml
Cl
-
4 mEq/100ml
K
+
2 mEq/100ml
Definition: Amount of fluid lost before treatment is
begun
One-time estimate; additional losses after therapy is
begun are considered on-going losses
Methods:
Preillness and current weight change
Fluid deficit (L) = Preillness weight (kg) current weight (kg)
% Dehydration = (Fluid deficit (L)/Preillness weight (kg))x100
Clinical estimates of weight loss
Sodium: usually in pediatrics, losses are
gastrointestinal or due to a relatively short period of
decreased oral intake
approximated by 0.45 NS
Potassium: deficit replacement is based on rate of
safe replacement and not amount since danger of
hyperkalemia is greater than hypokalemia
Add 20 mEq potassium/L after UOP is established
Potassium infusion rate should not exceed 1 mEq/kg/hour
unless in monitored setting
Fluid: abnormal losses that occur after the
one-time determination of a deficit
Diarrhea, vomiting, NG aspirates, polyuria
Measured and replaced cc for cc
Electrolytes:
Consult tables for electrolyte composition of on-going
losses
GI losses = 0.45 NS
Transudates = 0.9 NS
Radiant losses = sodium free
Maintenance fluid requirements must be modified according to the
childs clinical condition.
All types of fluid intake and output must be measured .
If the child is dehydrated or has excessive fluid losses, fluid intake
must be increased. For zero fluid balance, fluid losses = fluid intake.
Insensible fluid loss is fluid lost from the body in perspiration and
breathing, and is proportional to body surface area (BSA).
It is approximately 300ml/m2/day ,slightly higher in infants and
young children, warm temperature, pyrexia, tachypnoea, etc.
Using indirect calorimetric measurements, energy
expenditure in critically ill children may be as low as
50-60 kcal/kg/day.
Mechanical ventilation decreases the work of breathing
as well as evaporative water loss through the
respiratory tract and the energy expenditure for
thermal regulation.
Warmed humidification of respiratory gases through
the ventilator circuit can reduce insensible water losses
by as much as one third.
Hence, traditional estimates for maintenance fluid
volumes particularly in critically ill children cannot be
quantified from these general guidelines.
The most potent stimuli for ADH secretion are an increase In serum
osmolality, hypovolemia and hypotension. However, multiple
nonosmotic stimuli such as pain, drugs and anesthetic agents, stress,
and even nausea and vomiting may also result in increased ADH
activity.
There will be very little if any excretion of EFW, as ADH limits renal
water excretion in this setting, even in the presence of a low plasma
osmolality.
As a result, hyponatremia occurs due to a positive balance of EFW in
association with an impaired ability to excrete hypotonic urine.
Any exogenous sources of free water, such as the administration of
hypotonic IV maintenance fluids, will therefore further exacerbate the
fall in plasma sodium (PNa).
Recently conducted systematic review of
maintenance fluids for hospitalized children
revealed that the use of hypotonic fluids
remarkably increased the odds of developing
hyponatremia by 17 times when compared
to isotonic fluids.
Interstitial fluid 10.5 Litres
Blood volume 3.5 litres
Cells 28 Litres
Vasoconstriction &
redistribution
Interstitial fluid
mobilisation
Reduced
interstitial fluid
Intracellular fluid
mobilisation
Reduced intracellular fluid
What are we trying to achieve by giving
intravenous fluid ?
Scenario: Acute blood loss
Replacement of RBCs, water and
electrolytes - haemostasis
Inflammation
Inflammatory
cytokines
Neutrophils
Systemic
capillary leak
Leak of Water, Na
+
Cl
-
Albumin to Interstitium
Vasodilatation loss of SVR
Hypovolaemia
Interstitial
oedema
Interstitial
oedema
Na
+
Cl
-
water
Na
+
Cl
-
water
Na
+
and Cl
-
Loading
Fluid retention
Severe interstitial oedema
Organ dysfunction
What are we trying to achieve by giving
intravenous fluid ?
Scenario: Acute inflammation
Blood volume expansion, in the context of
vascular dysfunction and leaky capillaries
It is very easy to give salt & water to critically ill
patients, and very difficult to remove
Urine electrolyte measurement is essential for fluid
management in the critically ill
Electrolytes are salts, acids, and bases, but
electrolyte balance usually refers only to salt
balance
Salts are important for:
Neuromuscular excitability
Secretory activity
Membrane permeability
Controlling fluid movements
Salts enter the body by ingestion and are lost via
perspiration, feces, and urine
Expressed in milliequivalents per liter (mEq/L)
- a measure of the number of electrical charges in
one liter of solution
For monovalent ions, 1 mEq = 1 mOsm
For bivalent ions, 1 mEq = 1/2 mOsm
no. of electrical
charges on one
ion
mEq/L = (concentration of ion in [mg/L]
the atomic weight of ion
X
For monovalent ions, 1 Eq = 1 mole
For divalent ions, 1 Eq = 0.5 mol
For trivalent ions, 1 Eq = 0.333 mol
The equivalent (Eq or eq) is a measurement unit used in chemistry and the
biological sciences - a measure of a substance's ability to combine with
other substances - frequently used in the context of normality (GEW/liters
of solution)
The equivalent is defined as the mass (g) in grams of a substance which
will react with 6.022 x 10
23
electrons.
The equivalent weight of a substance is equal to the amount
in moles divided by the valence.
The exchange of interstitial and intracellular fluid is
controlled mainly by the presence of the electrolytes
sodium and potassium
Na
+
K
+
Na
+
K
+
Na
+
K
+
Na
+
K
+
Potassium is the chief intracellular cation and
sodium the chief extracellular cation
Because the osmotic pressure of the interstitial
space and the ICF are generally equal, water
typically does not enter or leave the cell
K
+
Na
+
A change in the concentration of either
electrolyte will cause water to move into or
out of the cell via osmosis
A drop in potassium will cause fluid to leave
the cell whilst a drop in sodium will cause fluid
to enter the cell
K
+
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
K
+
K
+
K
+
Na
+
Na
+
Na
+
Na
+
Aldosterone, ANP and ADH regulate sodium
levels within the body, whilst aldosterone can
be said to regulate potassium
K
+
Na
+
aldosterone
ADH
ANP
Sodium (Na
+
) ions are the important cations in
extracellular fluid
Anions which accompany sodium are chloride (Cl
-
)
and bicarbonate (HCO
3
-
)
Considered an indicator of total solute concentration
of plasma osmolality
Na
+
HCO
3
-
Cl
-
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Sodium ions are osmotically
important in determining water
movements
A discussion of sodium must also
include
Chlorine
Bicarbonate
Hydrogen ions
Potassium and calcium serum
concentrations are also important
electrolytes in the living system
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Click
Hypercalcemia - elevated calcium levels
Hypokalcemia -- lowered calcium levels
Click
Hyperkalemia -- elevated potassium levels
Hypokalemia ---- lowered potassium levels
Hypernatremia - elevated sodium levels
Hyponatremia -- lowered sodium levels
Participates in the Na-K pump
Assists in maintaining blood volume
Assists in nerve transmission & muscle contraction
Primary determinant of ECF concentration
Controls water distribution throughout the body
Primary regulator of ECF volume
Regulations: skin, GIT,Aldosterone increases Na retention
in the kidney
Normal range for blood levels of sodium is app. 137
- 143 meq/liter
Hypernatremia refers to an elevated serum sodium
level (145 -150 mEq/liter)
Increased levels of sodium ions are the result of
diffusion and osmosis
Na
+
1) Sodium ions do not cross cell membranes as
quickly as water does
Na
+
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Na
+
2) Cells pump sodium ions out of the cell by
using sodium-potassium pumps
Na
+
Na
+
Na
+
Na
+
3) Increases in extracellular sodium ion levels do
not change intracellular sodium ion concentration
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
1) Water is osmotically drawn out of the cells
Resulting in dehydration
2) Increase in extracellular fluid volume
Extracellular
fluid
volume
Intracellular
fluid
volume
In the CNS tight junctions exist between
endothelial cells of the capillary walls
These junctions restrict diffusion from
capillaries to the interstitium of the brain
blood-brain barrier
Increased levels of sodium ions in the
blood does not result in increased sodium
ions in brain interstitial fluid
As the result of an osmotic gradient, water
shifts from the interstitium and cells of the
brain and enters the capillaries
The brain tends to shrink and the capillaries
dilate and possibly rupture
Result is cerebral hemorrhage, blood clots,
and neurological dysfunction
H
2
O
There is an unknown mechanism that
protects the brain from shrinkage
Within about 1 day
Intracellular osmolality of brain cells increases in
response to extracellular hyperosmolality
Idiogenic osmoles accumulate inside brain cells
K
+
, Mg
+
from cellular binding sites and amino acids from
protein catabolism
These idiogenic osmoles create an osmotic force that
draws water back into the brain and protects cells
from dehydration
H
2
O
1) Water loss
2) Sodium ion overload
Most cases are due to water deficit
due to loss or inadequate intake
Infants without access to water or
increased insensible water loss can
be very susceptible to hypernatremia
Diabetes insipidus caused by inadequate ADH or
renal insensitivity to ADH results in large urinary
fluid loss
Increased fluid loss also occurs as the result of
osmotic diuresis (high solute loads are delivered to
the kidney for elimination)
Glucose
Glucose
Glucose
diabetes mellitus results in loss of fluids as well by
creating an osmotic pull (increased urine solute
concentration) on water into the tubules of the
kidney
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Glucose
Glucose
Glucose
diabetes mellitus results in loss of fluids as
well by creating an osmotic pull (increased
urine solute concentration) on water into the
tubules of the kidney
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Glucose
Glucose
Glucose
diabetes mellitus results in loss of fluids as
well by creating an osmotic pull (increased
urine solute concentration) on water into the
tubules of the kidney
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Glucose
Glucose
Glucose
diabetes mellitus results in loss of fluids as
well by creating an osmotic pull (increased
urine solute concentration) on water into the
tubules of the kidney
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Glucose
Glucose
Glucose
diabetes mellitus results in loss of fluids as
well by creating an osmotic pull
(increased urine solute concentration) on
water into the tubules of the kidney
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Glucose
Glucose
Glucose
diabetes mellitus results in loss of fluids as
well by creating an osmotic pull
(increased urine solute concentration) on
water into the tubules of the kidney
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Glucose
Glucose
Glucose
diabetes mellitus results in loss of fluids as
well by creating an osmotic pull (increased
urine solute concentration) on water into the
tubules of the kidney
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Glucose
Glucose
Glucose
diabetes mellitus results in loss of fluids as
well by creating an osmotic pull (increased
urine solute concentration) on water into
the tubules of the kidney
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Glucose
Glucose
Glucose
diabetes mellitus results in loss of fluids as
well by creating an osmotic pull (increased
urine solute concentration) on water into
the tubules of the kidney
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
Glucose
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Re-animate
High protein feedings by a stomach tube create high
levels of urea in the glomerular filtrate producing an
osmotic gradient the same as glucose does and
increased urinary output results
Occurs less frequently than water loss
Retention or intake of excess sodium
ex: IV infusion of hypertonic sodium ion solutions
Aldosterone promotes sodium and water
retention by the kidney
High levels of aldosterone may result in mild
hypernatremia
CAUSE COMMENTS
essent i al hyper nat r emi a di sor der i n whi ch t hi r st i s i mpai r ed
fever i ncr eased i nsensi bl e fl ui d l oss
coma i nadequat e fl ui d i nt ake
hot envi r onment , or st r enuous
exer ci se
sweat , hypot oni c fl ui d l oss
vomi t i ng oft en a hypot oni c fl ui d l oss
di ar r hea oft en a hypot oni c fl ui d l oss
pi t ui t ar y di abet es i nsi pi dus defi ci ency of ADH; excessi ve fl ui d
l oss
nephr ogeni c di abet es mel l i t us r enal t ubul es i nsensi t i ve t o ADH;
excessi ve ur i nar y l oss
uncont r ol l ed di abet es mel l i t us gl ucose i n gl omer ul ar fi l t r at e;
osmot i c di ur esi s
l ar ge amount s of pr ot ei n and ami no
aci ds gi ven by nasogast r i c t ube
ur ea i s a pr oduct of pr ot ei n
met abol i sm; ur ea causes osmot i c
di ur esi s
excessi ve i nt r avenous i nfusi on of
hyper t oni c sodi um sal t sol ut i ons
admi ni st r at i on of excessi ve sodi um
i ons
manni t ol used as di ur et i c manni t ol i n gl omer ul ar fi l t r at e;
osmot i c di ur esi s
Re-hydration is the primary objective in most cases
Decreases sodium concentrations
A point of concern is when
and how rapid the
re-hydration occurs
After 24 hours the brain has responded by
producing idiogenic osmoles to re-hydrate brain
cells
If this adaptation has occurred and treatment
involves a rapid infusion of dextrose for
example:
There is danger of cerebral
edema with fluid being
drawn into brain tissues
Treatment is best handled by giving slow
infusions of glucose solutions
This dilutes high plasma
sodium ion concentrations
Ideally the goal is to avoid overloading with fluid
and to remove excess sodium
Diuretics can be used to induce sodium and water
diuresis
However if kidney function is not normal peritoneal dialysis
may be required
Two pronged approach:
1. Identify and treat the underlying cause.
2 .Correct osmolar imbalance by replacing
what was lost (water, hypotonic fluids +/-
electrolytes) or ridding the body of excess
sodium
Hypovolemic:
Low total body Na, orthostasis: restore hemodynamics
with NS, then change to D5W or NS
Hypervolemic:
Excess total body Na. Give loop diuretics to increase Na
excretion and then replace D5W to correct
hypertonicity. Dialyze if kidneys are not working.
Euvolemic:
Normal total body Na. Give D5W.
If the Na has risen over a matter of <12 hours,
it can be correctly quickly without
consequence.
If elevated for longer than 12 hours or if the
onset is unclear, decrease Na by no more than
10 mmol/L/day or 0.5 mmol/L/hr.
Goal is 145.
One approach:
1. Calculate the total body water (TBW): 0.6 x (wt in
kg)
2. Select your fluid and identify the amount of Na in
mmol/L
D5W 0
NS 34
NS 77
LR 130
NS 154
3--Calculate the effect of 1 L of your selected
fluid on serum Na according to this formula:
Change in serum Na for 1L of fluid of choice
=[IVF Na - serum Na] divided by [TBW + 1]
If you are also giving K in your IVF, modify the
formula as follows:
Change in serum Na for 1L of fluid of choice
=[(IVF Na + IVF K) - serum Na] divided by
[ TBW + 1]
4-Decide how quickly you want to correct.
In cases of prolonged hypernatremia, divide 10 (the
desired drop) by the number obtained above to
calculate the amount of IVF required over the next 24
hours to decrease the serum Na appropriately.
When hypernatremia has been shorter-lived, divide the
number necessary to reach 145 by the number of hours
over which you want to correct.
5-Account for average obligatory 24 hour water losses
(1.5L or so)
6-Convert to mL and divide by 24 to obtain mL/hour
Defined as a serum sodium ion
level that is lower than normal
Implies an increased ratio of
water to sodium in extracellular
fluid
Extracellular fluid is more dilute
than intracellular fluid
Results in a shift of water into cells
Brain cells lose osmoles creating a higher
extracellular solute concentration
Effect is to protect against cerebral edema by
drawing water out of the brain tissue
Suppression of thirst
Suppression of ADH secretion
Both favor decreasing water
ingestion and increasing
urinary output
Primarily neurological (net flux of water into
the brain)
Sodium ion levels of 125 meq / liter are
enough to begin the onset of symptoms
Sodium ion levels of less than 110 meq / liter
bring on seizures and coma
Produced by:
1) A loss of sodium ions
2) Water excess
Water excess can be due to:
Ingestion
Renal retention
1) Isotonic fluid loss
2) Antidiuretic hormone secretion
3) Acute or chronic renal failure
4) Potassium ion loss
5) Diuretic therapy
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Na
+
Na
+
Na
+
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
Na
+
Na
+
Na
+
Na
+
Na
+
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
1) Isotonic fluid loss
Burns, fever, hemorrhage
Indirect cause of hyponatremia
Any volume loss stimulates thirst and leads to
increased water ingestion
Thus isotonic fluid loss can cause hyponatremia not
because of sodium loss but because of increased water
intake
2) Antidiuretic hormone secretion
Enhances water retention
3) Acute or chronic renal failure
The kidney fails to excrete water
Can lead to hyponatremia
4) Potassium ion loss
Potassium ions are the predominant intracellular
cations
When they are lost they are replaced by diffusion of
intracellular potassium into extracellular fluid
Electrical balance is maintained by the diffusion of
sodium ions into the cells in exchange for potassium
ions
Thus a loss of extracellular sodium is realized and
hyponatremia may ensue
K
+
Na
+
Na
+
K
+
K
+
K
+
1) extracellular
potassium loss
2) diffusion of potassium
ions into extracellular
compartments
3) intracellular electrical balance
is maintained by diffusion of
sodium ions into cells
plasma
interstitial fluid
cell
K
+
Na
+
K
+
K
+
K
+
Plasma
Interstitial fluid
Cell
K
+
K
+
Na
+
Na
+
Click to see
animation
5) Diuretic therapy
Common cause of hyponatremia
Loss of sodium and potassium often occurs in
addition to fluid loss
CAUSE COMMENTS
psychogeni c pol ydi psi a excessi ve i ngesti on of water
syndrome of i nappropri ate
secreti on of ADH
ADH causes renal water
retenti on
Addi son s di sease al dosterone defi ci ency
K
+
l osses from extracel l ul ar
fl ui d
K
+
move out of cel l s to repl ace
l osses; Na
+
move i nto cel l s to
mai ntai n el ectri cal neutral i ty
Increased Na
+
Osmoreceptors
inhibited
Decreased ADH
release
Decreased Thirst
Increased urinary
H
2
O loss
Decreased H
2
O gain
Decreased Na
+
Homeostasis
Normal Na
+
Osmoreceptors
stimulated
Increased ADH
release
Increased Thirst
Additional H
2
O
dilutes Na
+
H
2
O loss
concentrates Na
+
Decreased urinary
H
2
O loss
Increased H
2
O gain
10
10
60
K
+
Normal serum potassium level (3-5 meq /
liter)
As compared to Na
+
(142 meq / liter)
Intracellular levels of potassium (140-150
meq / liter)
This high intracellular level is maintained by active
transport by the sodium-potassium pump
K
+
Cells pump K
+
ions in and Na
+
ions out of the
cell by using sodium-potassium pumps
Na
+
Na
+
Na
+
Na
+
K
+
K
+
K
+
K
+
Hyperkalemia is an elevated serum potassium
(K
+
) ion level
A consequence of hyperkalemia is acidosis
an increase in H
+
ions in body fluids
Changes in either K
+
or H
+
ion levels causes a
compartmental shift of the other
K
+
When hyperkalemia develops potassium ions
diffuse into the cell
This causes a movement of H
+
ions out of the cell to
maintain a neutral electrical balance
As a result the physiological response to
hyperkalemia causes acidosis
K
+
K
+
K
+
K
+
K
+
K
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
HYPERKALEMIA
The reverse occurs as well
The body is protected from harmful effects of
an increase in extracellular H
+
ions (acidosis)
H
+
ions inside the cells are tied up by proteins
(Pr
-
)
This causes a shift of potassium ions out of
the cells
The reverse occurs as well
The body is protected from harmful effects of
an increase in extracellular H
+
ions (acidosis)
H
+
ions inside the cells are tied up by proteins (Pr
-
)
This causes a shift of potassium ions out of
the cells
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
K
+
K
+
K
+
K
+
K
+
K
+
ACIDOSIS
Summarized:
Hyperkalemia causes acidosis
Acidosis causes hyperkalemia
HYPERKALEMIA
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
K
+
K
+
K
+
K
+
K
+
K
+
K
+
K
+
ACIDOSIS
Summarized:
Hyperkalemia causes acidosis
Acidosis causes hyperkalemia
HYPERKALEMIA
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
K
+
K
+
K
+
K
+
K
+
K
+
K
+
K
+
ACIDOSIS
Muscle contraction is affected by
changes in potassium levels
Hyperkalemia blocks the
transmission of nerve impulses along
muscle fibers
Causes muscle weakness and paralysis
Can cause arrhythmia's and heart
conduction disturbances
1) Increased input of potassium
2) Impaired excretion of potassium
3) Impaired uptake of potassium by cells
A) Intravenous KCl infusion
B) Use of K
+
containing salt substitutes
C) Hemolysis of RBC during blood transfusions
with release of K
+
D) Damaged and dying cells release K
+
Burns, crush injuries, ischemia
E) Increased fragility of RBC
Insulin deficiency predisposes an
individual to hyperkalemia
Cellular uptake of K
+
ions is enhanced
by insulin, aldosterone and epinephrine
Provides protection from extracellular K
+
overload
Insulin
K
+
K
+
K
+
K
+
K
+
K
+
Click to view
animation
Insulin deficiency represents decreased
protection if the body is challenged by
an excess of K
+
ions
In the absence of aldosterone there is
loss of Na
+
in the urine and renal
retention of K
+
Inherited disorder in which serum K
+
level
rise periodically
Caused by a shift of K
+
from muscle to blood
in response to ingestion of potassium or
exercise
Reasons for the shift are
not clear
Attacks are characterized
by muscle weakness
Aldosterone has a primary role in promoting:
Conservation of Na
+
Secretion of K
+
by the nephrons of the kidney
Addisons disease is characterized by
aldosterone deficiency
Thus the kidney is unable
to secrete potassium at a
normal rate
Kidney loses the ability to secrete K
+
Diuretic that is antagonistic to the effects
of aldosterone
Causes some rise in serum K
+
levels by
interfering with K
+
secretion in the kidneys
Increases may not be significant
But individuals taking the
diuretic are at risk if
potassium is administered
1) Counteract effects of K
+
ions at the
level of the cell membrane
2) Promotion of K
+
ion movements into
cells
3) Removal of K
+
ions from the body
Infusion of calcium gluconate or NaCl
solutions
Immediately counteract the effects of K
+
ions on the heart
Effective for only 1-2 hours
NaHCO
3
also reverses hyperkalemic
effects on the heart
If acidosis is a factor also raises the pH
of body fluids
Insulin given with glucose
Effective in about 30 minutes
Has a duration of action of up to
6 hours
Insulin promotes the shift of K
+
ions into cells
Glucose prevents insulin-
induced hypoglycemia
Kayexalate (cation exchange resin)
Removes K
+
ions from the body by
exchanging K
+
for Na
+
Exchange time is about 45 minutes
Effective for up to
6 hours
Peritoneal dialysis or hemodialysis
Effectively clears the blood of high K
+
levels as well
CAUSE COMMENTS
hyper kal emi c per i odi c par al ysi s i nher i t ed di sor der i n whi ch t her e ar e sudden
shi ft s of cel l ul ar K+ t o ext r acel l ul ar
compar t ment s
aci dosi s compensat or y shi ft of H+ i nt o cel l s i n exchange
for movement of K+ t o ext r acel l ul ar
compar t ment s
bur ns cel l dest r uct i on wi t h r el ease of K+
t r ansfusi on of bl ood t hat has been
st or ed
r el ease of K+ fr om hemol yzed r ed bl ood cel l s
spi r onol act one di ur et i c t hat i s an al dost er one ant agoni st ;
i nt er fer es wi t h r eabsor pt i on of Na+ and
secr et i on of K+
t oo r api d i nt r avenous i nfusi on of KCl speci al r i sk of hyper kal emi a i f t hei r i s
i mpai r ed r enal secr et i on of K+
use of K+ cont ai ni ng sal t subst i t ut es excessi ve i ngest i on
pot assi um sal t s of ant i bi ot i cs addi t i onal sour ce of K+
acut e ol i gur i c r enal fai l ur e i mpai r ed secr et i on of K+
Defined as a serum K
+
level that is below
normal (< 3 meq / liter)
Serum concentrations will decrease if:
There is an intracellular flux of K
+
K
+
ions are lost from the gastrointestinal or
urinary tract
K
+
Alkalosis causes and is caused by
hypokalemia
Alkalosis is defined as a decrease of
hydrogen ions or an increase of
bicarbonate in extracellular fluids
Opposite of acidosis
K
+
H
+
HCO
3
-
Alkalosis elicits a compensatory response
causing H
+
ions to shift from cells to
extracellular fluids
This corrects the acid-base
imbalance
HCO
3
-
HCO
3
-
HCO
3
-
HCO
3
-
HCO
3
-
HCO
3
-
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
ions are exchanged for K
+
(potassium moves into cells)
Thus serum concentrations of K
+
are
decreased
And alkalosis causes
hypokalemia
HCO
3
-
HCO
3
-
HCO
3
-
HCO
3
-
HCO
3
-
HCO
3
-
H
+
H
+
H
+
H
+
H
+
H
+
H
+
H
+
K
+
K
+
K
+
K
+
K
+
K
+
K
+
K
+
Conversely when K
+
ions are lost from the
cellular and extracellular compartments
Sodium and hydrogen ions enter cells
in a ratio of 2:1 as replacement
This loss of extracellular H
+
causes alkalosis
HCO
3
-
H
+
K
+
HCO
3
-
HCO
3
-
HCO
3
-
HCO
3
-
HCO
3
-
HCO
3
-
H
+
H
+
H
+
H
+
H
+
H
+
Na
+
K
+
K
+
K
+
K
+
K
+
K
+
K
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Kidney function is altered by hypokalemia
Na
+
ions are reabsorbed into the blood when K
+
ions are secreted into the urine by kidney tubules
K
+
Tubular lumen
K
+
K
+
K
+
K
+
K
+
K
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Peritubular fluid
NORMAL
Kidney function is altered by hypokalemia
If adequate numbers of K
+
are not available for
this exchange
H
+
ions are secreted instead
H
+
Tubular lumen
K
+
H
+
K
+
H
+
K
+
H
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Na
+
Peritubular fluid
HYPOKALEMIA
Hypokalemia promotes renal loss of H
+
ions
and thus results in alkalosis
Normal nephron function
is to secrete H
+
and K
+
in
exchange for Na
+
capillary
distal tubule
H
+
K
+
Na
+
Blood
Urine
capillary
distal tubule
H
+
K
+
Na
+
In Hypokalemia the kidney
selectively secretes
H
+
ions in preference
to K
+
ions
The loss of H
+
ions
may lead to alkalosis
Blood
Urine
capillary
distal tubule
H
+
K
+
Na
+
retained
K
+
excreted
1) in alkalosis there is a
decrease in extracellular
fluid H
+
2) the kidney retains
hydrogen ions to
correct the alkalosis
3) the kidney then
eliminates K
+
ions
which can lead to
Hypokalemia
Blood
Urine
CAUSE COMMENTS
aldosterone excess favors renal Na+ reabsorption and K
+
secretion
diarrhea diarrheal fluid contains high amounts of K
+
diuretics in general causes K
+
loss
distal renal tubular
acidosis
kidney tubule defect in which K
+
are secreted, and H
+
are
retained by the body
hypokalemic periodic
paralysis
cause unknown; periodic influx of K
+
into cells
Bartters syndrome syndrome in which aldosterone is sometimes elevated;
probably a renal tubular defect so that K
+
are lost
Replacement of K
+
either by:
Oral K
+
salt supplements
Diet
Intravenous administration of K
+
salt
solution
Diuretic (spinolactone) if renal loss is
at work
Maximum IV infusion rate:
1 mEq/kg/hr
Marked hypokalemia:
Monitor serum K closely
0.5-1 mEq/kg/dose given as an infusion
of 0.5 mEq/kg/hr for 1-2 hour
You might also like
- A Simple Guide to Hypovolemia, Diagnosis, Treatment and Related ConditionsFrom EverandA Simple Guide to Hypovolemia, Diagnosis, Treatment and Related ConditionsNo ratings yet
- 2012-Fluid and ElectrolytesDocument192 pages2012-Fluid and ElectrolytesHarley Justiniani Dela CruzNo ratings yet
- Fluid and Electrolytes for Nursing StudentsFrom EverandFluid and Electrolytes for Nursing StudentsRating: 5 out of 5 stars5/5 (12)
- Fluids and ElectrolytesDocument192 pagesFluids and ElectrolytesTeodora JoghiuNo ratings yet
- Fluid Management and Obstetric ShockDocument188 pagesFluid Management and Obstetric Shocksprimal50% (2)
- Fluid and Electrolyte Imbalance PDFDocument21 pagesFluid and Electrolyte Imbalance PDFShafaq AlamNo ratings yet
- PP Prinsip Terapi Cairan Dan ElektrolitDocument56 pagesPP Prinsip Terapi Cairan Dan ElektrolitDedare Shop100% (1)
- Fluid Electrolyte and AcidBase BalanceDocument33 pagesFluid Electrolyte and AcidBase Balancemoncalshareen3No ratings yet
- Asam Basa Dan ElektrolitDocument50 pagesAsam Basa Dan ElektrolitDavid Ithu AgkhuNo ratings yet
- Fluids and Electrolytes NCM 101Document142 pagesFluids and Electrolytes NCM 101France John Evangelista Torres100% (2)
- Tutorial 1 - Muntaber: Melia Fadiansari S. 13-163 2B FK UkiDocument60 pagesTutorial 1 - Muntaber: Melia Fadiansari S. 13-163 2B FK UkiWibbyReksoNo ratings yet
- Kompartemen Cairan TubuhDocument27 pagesKompartemen Cairan Tubuhdalang leriNo ratings yet
- Kompartemen Cairan Tubuh (For PSPDG)Document27 pagesKompartemen Cairan Tubuh (For PSPDG)Riezka HanafiahNo ratings yet
- Fluid and Electrolyte BalanceDocument41 pagesFluid and Electrolyte BalanceFev BanataoNo ratings yet
- Fluid & Electrolyte ImbalancesDocument212 pagesFluid & Electrolyte ImbalancesLane Mae Magpatoc NoerrotNo ratings yet
- Fluid and Electrolytes (Acid Base)Document31 pagesFluid and Electrolytes (Acid Base)Diana TahamidNo ratings yet
- Fluid and Electrolyte-1Document37 pagesFluid and Electrolyte-1Roshini R KrishnanNo ratings yet
- Water Electrolytes Part Clinical BiochemistryDocument27 pagesWater Electrolytes Part Clinical BiochemistryAshraf FirdadNo ratings yet
- Fluid and ElectrolytesDocument102 pagesFluid and ElectrolytesBindiya MangarNo ratings yet
- Week 3Document396 pagesWeek 3Danica Mae BianitoNo ratings yet
- Intravenous Fluids: Composition & UsesDocument41 pagesIntravenous Fluids: Composition & UsesFathima BanuzNo ratings yet
- Pathophysiology Module 4Document26 pagesPathophysiology Module 4johnbryanmalonesNo ratings yet
- Fluid, Electrolyte, Acid Base BalanceDocument42 pagesFluid, Electrolyte, Acid Base BalanceSutrisno YangNo ratings yet
- NSG IV Test 3Document4 pagesNSG IV Test 3Maria Phebe SinsayNo ratings yet
- Fluid and ElectrolyteDocument66 pagesFluid and ElectrolyteMoni Mbumba Meleke100% (1)
- 1.1a Fluid Management 6th Seminar GRP AaDocument40 pages1.1a Fluid Management 6th Seminar GRP AahalearnNo ratings yet
- Fluid and ElectrolyteDocument46 pagesFluid and Electrolyteapi-76740522No ratings yet
- Fluids and ElectrolytesDocument71 pagesFluids and ElectrolytesHarold Castillo OrigenesNo ratings yet
- Lec. 3 - Fluid and ElectrolyteDocument16 pagesLec. 3 - Fluid and Electrolyteمجيب سلطانNo ratings yet
- Fluid ElectrolyteDocument115 pagesFluid ElectrolytePaul EbenezerNo ratings yet
- Fluid ManagementDocument10 pagesFluid ManagementdradaadNo ratings yet
- Fluid and Electrolyte BalanceDocument87 pagesFluid and Electrolyte Balancerajashree kokatanurNo ratings yet
- Intravenous Fluid Therapy: Part o NeDocument68 pagesIntravenous Fluid Therapy: Part o NeMourian AmanNo ratings yet
- Intake OutputDocument52 pagesIntake Outputkirti thakurNo ratings yet
- BODY Weight 100%: Balance/Imbalances & TherapyDocument11 pagesBODY Weight 100%: Balance/Imbalances & TherapyVictoria Castillo TamayoNo ratings yet
- Ivfluidtherapytypesindicationsdosescalculation 130123090523 Phpapp01Document68 pagesIvfluidtherapytypesindicationsdosescalculation 130123090523 Phpapp01rainNo ratings yet
- Dehydration 'Acid Base Balance DisordersDocument38 pagesDehydration 'Acid Base Balance DisordersMalika SadridinovaNo ratings yet
- Basic of Fluid Therapy ImaDocument69 pagesBasic of Fluid Therapy Imal Made ArtawanNo ratings yet
- Body Fluid 1Document55 pagesBody Fluid 1Anonymous z3afjyy1aNo ratings yet
- Overview of Fluids and ElectrolytesDocument24 pagesOverview of Fluids and ElectrolytesAira Jane BasuelNo ratings yet
- Fluid and Electrolyte Balance: Presenter: Dr. Siyum Mathewos (Omfs-Ri) Modulator: Dr. Dereje (Omfs, Consultant)Document87 pagesFluid and Electrolyte Balance: Presenter: Dr. Siyum Mathewos (Omfs-Ri) Modulator: Dr. Dereje (Omfs, Consultant)Siyum MathewosNo ratings yet
- Fluid and Electrolytes: Ma. Medine L. Amorsolo RN ManDocument29 pagesFluid and Electrolytes: Ma. Medine L. Amorsolo RN ManMichael Baylon Dueñas100% (1)
- Dasar Terapi Cairan Dan ElektrolitDocument23 pagesDasar Terapi Cairan Dan ElektrolitMonika Tatyana YusufNo ratings yet
- Intro To Fluid and Electrolytes 2022Document42 pagesIntro To Fluid and Electrolytes 2022David Dwane Art SilorioNo ratings yet
- Fluids ElectrolytesDocument37 pagesFluids ElectrolytesAlpascaFirdausNo ratings yet
- Fluid & Electrolyte Balance: Part 4: Regulation & MaintenanceDocument40 pagesFluid & Electrolyte Balance: Part 4: Regulation & MaintenanceMy MusicNo ratings yet
- Fluids & Electrolytes: A. 2 Body CompartmentsDocument5 pagesFluids & Electrolytes: A. 2 Body CompartmentsJULIUS ART VINCENT A. PADINITNo ratings yet
- DR Faiyaz PGT Su1 2/6/2015: BalanceDocument42 pagesDR Faiyaz PGT Su1 2/6/2015: BalanceRubinaNo ratings yet
- FLUID AND ELECTOLYTE IMBALANCE FinalDocument27 pagesFLUID AND ELECTOLYTE IMBALANCE FinalShiva BiradarNo ratings yet
- Pathophysiology (NUR 190) Carmen Corder, MSN, RN Fluids and Electrolyte BalanceDocument17 pagesPathophysiology (NUR 190) Carmen Corder, MSN, RN Fluids and Electrolyte BalanceSydney DeringNo ratings yet
- Fluid Volume BalanceDocument73 pagesFluid Volume BalanceSalman HabeebNo ratings yet
- Fluid, Electrolyte and Acid-Base BalanceDocument59 pagesFluid, Electrolyte and Acid-Base BalanceFlourence ZafranNo ratings yet
- Basic Renal NotesDocument4 pagesBasic Renal Notesyannie.s.liNo ratings yet
- Lect15&16 Fluids&ElectrolytesDocument77 pagesLect15&16 Fluids&Electrolyteskhurram na100% (1)
- Rudaina Alelyani MEV Assignment3.Document15 pagesRudaina Alelyani MEV Assignment3.Rudaina AlelyaniNo ratings yet
- Fluid, Electrolyte and Acid-Base BalanceDocument74 pagesFluid, Electrolyte and Acid-Base BalanceIgwe Solomon100% (1)
- Francis M. Albances, RN SPU Iloilo 2009Document126 pagesFrancis M. Albances, RN SPU Iloilo 2009Christian Lester Ramos PastorNo ratings yet
- Fluids and Electrolytes Pathophysiology NursingDocument16 pagesFluids and Electrolytes Pathophysiology Nursinggrad_nurse_2015100% (3)
- S.No Table of Content Page NoDocument20 pagesS.No Table of Content Page NoTamilArasiNo ratings yet
- Fluids & Electrolytes: April Love Rivera-Oja, RN-MANDocument56 pagesFluids & Electrolytes: April Love Rivera-Oja, RN-MANAdelin MeroNo ratings yet
- 2014 CHN Question Ares With Rationale 1Document15 pages2014 CHN Question Ares With Rationale 1kyuss2No ratings yet
- Orientation 2013 H. DelacruzDocument30 pagesOrientation 2013 H. Delacruzkyuss2No ratings yet
- 2014-2015 General OrientationDocument3 pages2014-2015 General Orientationkyuss2No ratings yet
- Pathognomonic Is A Sign or Symptom That Is So Characteristic of A Disease That It Makes The DiagnosisDocument1 pagePathognomonic Is A Sign or Symptom That Is So Characteristic of A Disease That It Makes The Diagnosiskyuss2No ratings yet
- A. B. C. DDocument2 pagesA. B. C. Dkyuss2No ratings yet
- Office of The Student Affairs: Saint Gabriel CollegeDocument6 pagesOffice of The Student Affairs: Saint Gabriel Collegekyuss2No ratings yet
- Problem Oriented ChartingDocument27 pagesProblem Oriented Chartingkyuss2No ratings yet
- Pathognomonic Is A Sign or Symptom That Is So Characteristic of A Disease That It Makes The DiagnosisDocument1 pagePathognomonic Is A Sign or Symptom That Is So Characteristic of A Disease That It Makes The Diagnosiskyuss2No ratings yet
- MCL Procedure For Waived ApplicationsDocument5 pagesMCL Procedure For Waived Applicationskyuss2No ratings yet
- Skill 103 Administering A Large-Volume Cleansing EnemaDocument3 pagesSkill 103 Administering A Large-Volume Cleansing EnemaHarley Justiniani Dela CruzNo ratings yet
- Ms New Lecture 2012Document67 pagesMs New Lecture 2012kyuss2No ratings yet
- Types Ofnasogastric TubesDocument1 pageTypes Ofnasogastric Tubeskyuss2No ratings yet
- MCN Midterm 2014Document79 pagesMCN Midterm 2014kyuss2No ratings yet
- Medical Mission Frank TalkDocument16 pagesMedical Mission Frank Talkkyuss2No ratings yet
- Lynn12 12Document2 pagesLynn12 12Harley Justiniani Dela CruzNo ratings yet
- Pharmacology Made EasyDocument151 pagesPharmacology Made Easykyuss2100% (4)
- SKILL 103 - Nasogastric-Tube 2012Document85 pagesSKILL 103 - Nasogastric-Tube 2012kyuss2100% (1)
- Pnle 2013 December TakersDocument45 pagesPnle 2013 December Takerskyuss2No ratings yet
- List of StudentsDocument1 pageList of Studentskyuss2No ratings yet
- 2012 July Examinees Room AssignmentDocument9 pages2012 July Examinees Room Assignmentkyuss2No ratings yet
- The Association of Nursing Service Administrators of The PhilippinesDocument2 pagesThe Association of Nursing Service Administrators of The Philippineskyuss2No ratings yet
- The Association of Nursing Service Administrators of The PhilippinesDocument2 pagesThe Association of Nursing Service Administrators of The Philippineskyuss2No ratings yet
- Skill103 Removal CatherizationDocument2 pagesSkill103 Removal Catherizationkyuss2No ratings yet
- Medical Mission Frank TalkDocument16 pagesMedical Mission Frank Talkkyuss2No ratings yet
- Advanced Primary Health NursingDocument24 pagesAdvanced Primary Health Nursingkyuss2No ratings yet
- 10 DiabetesDocument16 pages10 Diabeteskyuss2No ratings yet
- Endocrine NursingDocument16 pagesEndocrine NursingFreeNursingNotes100% (18)
- Method of Instruction:Lecture, Powerpoint, Reading, Discussion, Case StudyDocument3 pagesMethod of Instruction:Lecture, Powerpoint, Reading, Discussion, Case Studykyuss2No ratings yet
- Co-Dominance Blood Groups and Rhesus Factor: DR - Mohammed Iqbal Musani, MDDocument38 pagesCo-Dominance Blood Groups and Rhesus Factor: DR - Mohammed Iqbal Musani, MDisasai52No ratings yet
- Chapter 21 of Anatomy and Physiology by John Wiley and SonsDocument48 pagesChapter 21 of Anatomy and Physiology by John Wiley and SonsRonaldo ZapateroNo ratings yet
- Water LoseDocument29 pagesWater LoseJAKLIN EMPOLNo ratings yet
- Cleaning of Brain Cells by SoundDocument27 pagesCleaning of Brain Cells by SoundRadoslav Shterev100% (1)
- Hemodynamic Disorders, Thrombosis, and Shock GWAIDocument102 pagesHemodynamic Disorders, Thrombosis, and Shock GWAIkavindukarunarathnaNo ratings yet
- Water, ElectrolyteDocument15 pagesWater, Electrolytevicky_law_2No ratings yet
- Revision Notes.Document32 pagesRevision Notes.drhaj70% (1)
- Crystalloid-Colloid: Body FluidsDocument1 pageCrystalloid-Colloid: Body FluidsgopscharanNo ratings yet
- Prinsip HomeostasisDocument20 pagesPrinsip HomeostasisRobby PrasetyoNo ratings yet
- Clinical Kinesiology Web VersionDocument21 pagesClinical Kinesiology Web Versiondrhannibal32100% (1)
- Lymphatic CaseDocument6 pagesLymphatic CaseGlenn Arjay BataraoNo ratings yet
- Peripheral Edema: ReviewDocument7 pagesPeripheral Edema: ReviewVmsdNo ratings yet
- Chapter 29 Lymphatic System PDFDocument24 pagesChapter 29 Lymphatic System PDFMACON824No ratings yet
- Water BalanceDocument9 pagesWater BalanceArch Ricky SantosNo ratings yet
- Textbook Preface PDFDocument13 pagesTextbook Preface PDFRobert StevenNo ratings yet
- Telebrix Training 2005Document51 pagesTelebrix Training 2005monolith4200No ratings yet
- Dry Brushing TechniqueDocument3 pagesDry Brushing Techniqueapi-206772759100% (1)
- Non-Cardiogenic Pulmonary EdemaDocument7 pagesNon-Cardiogenic Pulmonary EdemadarmariantoNo ratings yet
- Lymphatic SystemDocument3 pagesLymphatic SystemKhusyairy Daeng SailendraNo ratings yet
- MCQs & EMQs in Human PhysiologyDocument361 pagesMCQs & EMQs in Human Physiologybrandonjm96100% (1)
- Body Fluids Compartments and ExchangeDocument21 pagesBody Fluids Compartments and ExchangeLIDIYA MOL P VNo ratings yet
- DR Karel JindrakDocument35 pagesDR Karel Jindrakpost2vishalNo ratings yet
- Ch16 Answer Key 12edition CorrectedDocument15 pagesCh16 Answer Key 12edition CorrectedGurpreetKainth100% (1)
- HSB QA What Is DigestionDocument37 pagesHSB QA What Is DigestionVivienne WrightNo ratings yet
- Regulating The Internal EnvironmentDocument9 pagesRegulating The Internal EnvironmentUmaira BalqisNo ratings yet
- EdemaDocument13 pagesEdemaNUR ZAMZAM AZIZAHNo ratings yet
- Fluid and Electrolyte Disorders: Dr. Chandra Kant Pandey Dr. R. B. SinghDocument8 pagesFluid and Electrolyte Disorders: Dr. Chandra Kant Pandey Dr. R. B. SinghMok Chu ZhenNo ratings yet
- Biology 2A03 Quiz 1 Version 1 Answers Feb 2013Document8 pagesBiology 2A03 Quiz 1 Version 1 Answers Feb 2013JeevikaGoyalNo ratings yet
- Blood Vessels NotesDocument5 pagesBlood Vessels NotesJan Vincent GoNo ratings yet
- From Cells To Systems Test Bank Chapter 1Document15 pagesFrom Cells To Systems Test Bank Chapter 1lucas pone67% (3)
- Chapter 20bDocument6 pagesChapter 20baexillisNo ratings yet