Professional Documents
Culture Documents
Arsenic Removal From Wastewater
Uploaded by
subho0 ratings0% found this document useful (0 votes)
57 views14 pagesA powerpoint presentation on arsenic removal from wastewater
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentA powerpoint presentation on arsenic removal from wastewater
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
57 views14 pagesArsenic Removal From Wastewater
Uploaded by
subhoA powerpoint presentation on arsenic removal from wastewater
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 14
What is Wastewater and why do we
need to treat it.
• Wastewater is basically used water. It includes substances such as human
waste, food scraps, oils, soaps and chemicals. In homes, this includes
water from sinks, showers, bathtubs, toilets, washing machines and
dishwashers. Businesses and industries also contribute their share of used
water that must be cleaned.
• If wastewater is not properly treated, then the environment and human
health can be negatively impacted.
• These impacts can include harm to fish and wildlife populations, oxygen
depletion, beach closures and other restrictions on recreational water use,
restrictions on fish and shellfish harvesting and contamination of drinking
water.
• A number of excessive nutrients can clog up water bodies, causing
eutrophication.
• Heavy metals such as arsenic are extremely harmful, and can have acute
and chronic toxic effects on a number of aquatic and land species.Hence
the very dire need to treat it.
Arsenic and its toxicity
• Arsenic is only one of the several metallic constituents present in
the various water sources.
• It mainly exists in the +3 and +5 oxidation states.
• It has been recognized by the World Health Organisation(WHO) as a
group 1 human carcinogenic substance.
• Upon intake of only a minute amount of it, the human body gets
attacked by a number of diseases, some of which might be for a
prolonged time.
• Studies have shown that compared to +5 oxidation state, the
element in its +3 oxidation state is much more harmful, as it is more
cytotoxic, genotoxic, mobile, and soluble.
• Therefore, it is absolutely vital that the toxic element be removed
or the wastewater cleaned up off it as far as possible, to make sure
that humans consuming the water, or the aquatic animals and
plants intaking the water contain as little of it as is permitted.
Major treatments to remove the
arsenic from waters
Oxidation:
• Involves the conversion of soluble arsenic to an insoluble one.
• The process however, requires further treatment methods, which might
include coagulation/flocculation, chemical precipitation, or ion exchange,
all of which will be discussed.
• In developing countries, atmospheric oxygen, hypochlorite, and
permanganate are the most commonly used oxidants.
• The process generally converts the arsenic in its +3 oxidation state to its +5
oxidation state, in which the substance is much more insoluble, which can
then be treated by using techniques such as adsorption and precipitation
to finally remove the arsenic from the water.
• Some compounds however, cannot be used satisfactorily as oxidants, for
example, ozone(O3) cannot be used if the water contains S2- or TOC.
Hence, it is necessary that oxidising materials be selected extremely
carefully for these processes.
Oxidants used to convert As(III) to
As(V)
• A study conducted has listed and found seven oxidants which can be successfully
used to a certain extent for the conversion of the As(III) to As(V).
• Chlorine has been found to be the most effective oxidant in the successful
conversion of As(III) to As(V), although the present of sulphide in the same water
hindered the success a bit.
• As in the case with chlorine, permanganate was found to be as effective , if not
faster, in the oxidation of As(III) to As(V).
• Ozone was again found to be another effective method in the conversion, and
whole process was completed in just under 15 seconds. But at the time, sulphide,
again hindered and slowed down the process to the minimal amount.
• Chlorine dioxide has been identified as another oxidant, although it was termed as
a huge failure, as it was found to help convert only a minimal amount of 20-30% of
the As(III).
• Preformed monochloramine, UV irradiation, and filox solid media, although
identified as oxidants, cannot be readily or successfully used in the oxidising
process, owing to their extremely low success rates.
• The next few slides talk about the various methods used to finally remove the
arsenic from the water after having been converted into the +5 oxidation state.
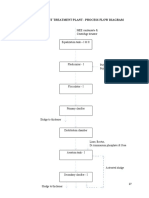
A Basic outline of the treatment process which shows the wastewater being treated by
oxidation followed by the previously mentioned physico-chemical processes.
• A major point to be noted in the removal of arsenic is that, , at a pH of
around 6.5-8.5, the element present as a compound in its +3 oxidation
state is generally uncharged, and in its reduced state. On the other hand,
the arsenic present as a compound in its +5 oxidation state is generally
anionic. Thus from the general point of view, the arsenic in its +5 oxidation
state is much easier to remove as it is in a much more unstable state than
the compounds containing arsenic in its +3 oxidation state. Anion
exchange and adsorption and precipitation can therefore be readily used
to remove the arsenic once it is in its +5 oxidation state.
• Thus it becomes extremely necessary that if the arsenic is present in its +3
oxidation state, that it should initially be have to be converted to its +5
oxidation state, before any of the major treatment techniques be carried
out.
• The following slide will discuss the various techniques and compounds
used to remove the arsenic from the water once it has been converted
into the +5 oxidation state.
Coagulation/Flocculation
• Coagulation is a process of treating water by using coagulants like
alum to neutralize the charges on the colloidal particles, thus
binding them together and forming lumps much bigger in size as
compared to the colloids, making it easy to separate the needed
constituents from the water.
• In the removal of arsenic from wastewater, iron salts are used as
coagulants, which helps remove more tan 90% of arsenate from the
water, at a pH of around 7, whereas at a pH level of above 7, flocs
help remove arsenic extremely effectively.
• The iron salts help remove around 50% of the trivalent(+3) arsenic
from the water.
• Some iron and manganese are removed from the water using a
combination of oxidation and precipitation, along with the arsenic,
which is generally adsorbed.
Pureflow coagulation/filtration system at the
Salt Lake Service Area #3 (Snowbird, UT)
drinking water plant
Ion Exchange processes
• It is basically a process which has been in use for a pretty
long time, and involves the use of resins(in this case a
strong base one) to exchange the anions on the arsenic
compound with those of it.
• Strong base resins permit the use of ordinary sodium
chloride brine for regeneration, and eliminate the need for
the use of strong acids.
• An extremely effective method, this helps in producing
what we call today, deionised water.
• The chlorine ions get replaced by the arsenic ions in the
exchange sites, while the arsenic ones present in the water
gets exchanged and is converted into the harmless chloride
ions.
Activated Alumina
• Activated alumina has a long history of use as an adsorptive treatment
technology for arsenic removal.
• The media is a byproduct of aluminum production. It primarily is an
aluminum oxide that has been activated by exposure to high temperature
and caustic soda.
• The material is extremely porous and has a high average surface area per
unit weight.
• The capacity for arsenic removal by activated alumina is pH-dependent,
with the maximum removal capacity achieved at pH 5.5. Adjusting the pH
of the source water, therefore, provides removal capacity advantages.
• The process, however, is preferential for arsenic at the optimum pH level
of 5.5. Thus the process is extremely pH dependent, and at pH not
pertaining to 5.5, it is difficult to remove arsenic. Similarly, at a pH of 5.5,
the other constituents present in the water cannot be removed with the
same rate of success.
The basic membrane separation
processes
• Electrodialysis, reverse osmosis are the common and
the widely known technologies which have been in use
for a long time, to remove arsenic and other harmful
heavy metals from the wastewater, as well as other
water sources, where reverse osmosis uses a semi
permeable membrane and pressure differences for the
treatment of water.
• Lime softening too, is used at many industrial facilities,
although the initial requirements for this is the removal
calcium and magnesium salts from the salts from the
water, thus making the water soft.
The first picture shows an electrolysis process of separation
And the second shows a lime softener plant.
Emerging Treatment Technologies
• Several innovative arsenic removal technologies, or variations of
existing technologies, have been developed over the past decade.
• ElectroChemical Arsenic Remediation (ECAR) uses a low electrical
current to create rust from iron plates in contaminated water. The
rust binds to arsenic, which can then be removed from the water
through settling and/or filtration.
• Regenerating adsorptive media (AM) is another recent
development. A caustic soda solution (pH 13) is used to strip arsenic
off the media.
• Another low-cost technology designed to address arsenic removal
in developing countries is subterranean arsenic removal (SAR). This
technology uses controlled oxidation with air and filtration to
reduce arsenic to low levels.
THANK YOU.
You might also like
- Municipal Wastewater Treatment: Evaluating Improvements in National Water QualityFrom EverandMunicipal Wastewater Treatment: Evaluating Improvements in National Water QualityNo ratings yet
- Water Treatment Plant Performance Evaluations and OperationsFrom EverandWater Treatment Plant Performance Evaluations and OperationsNo ratings yet
- Water Treatment I Kurang KomplitDocument89 pagesWater Treatment I Kurang KomplitEndarAdeCandraNo ratings yet
- Training Manual For Water TreatmentDocument181 pagesTraining Manual For Water Treatmentmuhammad abdulrehmanNo ratings yet
- Water TreatmentDocument30 pagesWater TreatmentDurga PrasadNo ratings yet
- Systematic Methods of Water Quality Parameters Analysis: Analytical MethodsFrom EverandSystematic Methods of Water Quality Parameters Analysis: Analytical MethodsNo ratings yet
- Boiler Water Treatment: Deposit ControlDocument5 pagesBoiler Water Treatment: Deposit ControlKrishna RayuduNo ratings yet
- Wastewater Treatment Methods ExplainedDocument45 pagesWastewater Treatment Methods ExplainedHamza RiazNo ratings yet
- Advanced Wastewater Treatment and Sludge ManagementDocument52 pagesAdvanced Wastewater Treatment and Sludge ManagementFrancis TiehNo ratings yet
- Arsenic Removal From WaterDocument53 pagesArsenic Removal From WaterSachin BansalNo ratings yet
- Wastewater TrainDocument3 pagesWastewater TrainLester Mercado100% (1)
- Wastewater Treatment: A 3-Stage Process for Cleaning WaterDocument37 pagesWastewater Treatment: A 3-Stage Process for Cleaning WaterGeofrey S BajeNo ratings yet
- Lesson Plan: How Do We Clean Polluted Water?Document15 pagesLesson Plan: How Do We Clean Polluted Water?Tarun MattaparthyNo ratings yet
- Experiment 3 Arvia Water TreatmentDocument24 pagesExperiment 3 Arvia Water TreatmentBrendaNo ratings yet
- Lamella Clarifier Leopold TexlerDocument4 pagesLamella Clarifier Leopold TexlerAntony ThanosNo ratings yet
- Demineralized Water Purity GuideDocument1 pageDemineralized Water Purity GuideeltonNo ratings yet
- Usuma Dam Water Treatment PlantDocument16 pagesUsuma Dam Water Treatment PlantAisha SaeedNo ratings yet
- TC Owtu 502 PDFDocument288 pagesTC Owtu 502 PDFRon100% (1)
- Nitrogen RemovalDocument7 pagesNitrogen RemovalvukoNo ratings yet
- Techniques For Waste Water TreatmentDocument50 pagesTechniques For Waste Water TreatmentManu JainNo ratings yet
- Water Treatment Processes: Disinfection and Removal of SolidsDocument24 pagesWater Treatment Processes: Disinfection and Removal of SolidsDhana BhargavNo ratings yet
- Manganese Greensand PlusDocument4 pagesManganese Greensand PlusPT Purione MegatamaNo ratings yet
- 16 - Residual Chlorine and Chlorine DemandDocument29 pages16 - Residual Chlorine and Chlorine DemandHayden Chappelear-RobbinsNo ratings yet
- Classified - Internal UseDocument18 pagesClassified - Internal UseNandha KumarNo ratings yet
- Ion Exchange Regeneration Methods PDFDocument10 pagesIon Exchange Regeneration Methods PDFDeblina MukherjeeNo ratings yet
- Ultra Pure WaterDocument6 pagesUltra Pure Watergauravgarg115No ratings yet
- Operating A Water Treatment Plant Is Complex and Requires Knowledge of MachineryDocument12 pagesOperating A Water Treatment Plant Is Complex and Requires Knowledge of MachineryJoshua OmolewaNo ratings yet
- WATER TREATMENT TECHNOLOGY (TAS 3010) LECTURE NOTES 9e - DisinfectionDocument17 pagesWATER TREATMENT TECHNOLOGY (TAS 3010) LECTURE NOTES 9e - Disinfectionmamat88No ratings yet
- 2010 SMR ClarifierDocument60 pages2010 SMR ClarifierRohan KakdeNo ratings yet
- WEEM 3510 Water and Wastewater Lab ManualDocument65 pagesWEEM 3510 Water and Wastewater Lab ManualMichael Huisa Taipe100% (1)
- Water Treatment Chemicals ListDocument7 pagesWater Treatment Chemicals ListAlvin KimNo ratings yet
- Water Treatment Processes - Coagulation and Flocculation ExplainedDocument7 pagesWater Treatment Processes - Coagulation and Flocculation ExplainedDr-Manoj GargNo ratings yet
- Topic 6 - Water TreatmentDocument57 pagesTopic 6 - Water TreatmentYousef ZamNo ratings yet
- Softening: Water TreatmentDocument20 pagesSoftening: Water Treatmentpkgarg_iitkgpNo ratings yet
- Removal of Iron From GroundwaterDocument3 pagesRemoval of Iron From GroundwaterRishya Prava ChatterjeeNo ratings yet
- Co2 Degasifier To Adjust The PHDocument2 pagesCo2 Degasifier To Adjust The PHGhuna UcihaNo ratings yet
- Anaerobic Digestion of Waste Activated S PDFDocument23 pagesAnaerobic Digestion of Waste Activated S PDFnawajhaNo ratings yet
- Wastestabilization PondsDocument29 pagesWastestabilization PondsKamukwema johnNo ratings yet
- Nitrates Removal StrategiesDocument12 pagesNitrates Removal Strategiesharoon_siyech_enggNo ratings yet
- Gas Chlorine StationsDocument46 pagesGas Chlorine StationsakramhomriNo ratings yet
- 07 - Lime SofteningDocument4 pages07 - Lime SofteningRAJ_1978No ratings yet
- Scale Control OverviewDocument22 pagesScale Control OverviewReda Abdel AzimNo ratings yet
- DBKK Industrial Training Presentation on Wastewater Treatment ProcessesDocument20 pagesDBKK Industrial Training Presentation on Wastewater Treatment ProcesseslooperinNo ratings yet
- Wastewater TreatmentDocument25 pagesWastewater TreatmentLTE002No ratings yet
- Chlorine FAODocument289 pagesChlorine FAOTrầnĐứcHùngNo ratings yet
- Effluent Treatment Plant - Process Flow DiagramDocument45 pagesEffluent Treatment Plant - Process Flow DiagramAmarnath PNo ratings yet
- Unit 5 PDFDocument154 pagesUnit 5 PDFKhalilNo ratings yet
- Sludge Treatment and DisposalDocument30 pagesSludge Treatment and DisposalIndrajeet UpadhyayNo ratings yet
- Chapter 5Document23 pagesChapter 5Tefera TemesgenNo ratings yet
- Monitoring, Operation and Control of Ion Exchange Plant: Mel HallDocument73 pagesMonitoring, Operation and Control of Ion Exchange Plant: Mel HallefasaravananNo ratings yet
- Brian Windsor Troubleshooting1Document35 pagesBrian Windsor Troubleshooting1brandlabBDNo ratings yet
- Remove contaminants & purify waterDocument13 pagesRemove contaminants & purify watermister_no34No ratings yet
- Introduction BODDocument4 pagesIntroduction BODShaoline LungaoNo ratings yet
- SIDE STREAM FILTRATION HIGH CAPACITY AND LOW FLUSH WATER LOSSDocument1 pageSIDE STREAM FILTRATION HIGH CAPACITY AND LOW FLUSH WATER LOSSAmit ChaudharyNo ratings yet
- Water DemineralizationDocument3 pagesWater DemineralizationHazel Marie ArceñoNo ratings yet
- Denitrification ProcessDocument3 pagesDenitrification Processmishti3001No ratings yet
- A Presentation On Mean Value TheoremDocument22 pagesA Presentation On Mean Value TheoremsubhoNo ratings yet
- Verbal Ability (CAT)Document9 pagesVerbal Ability (CAT)Anonymous Vx9KTkM8nNo ratings yet
- Types of Radioactive WasteDocument3 pagesTypes of Radioactive WastesubhoNo ratings yet
- 6th Central Pay Commission Salary CalculatorDocument15 pages6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- Chemical Proces-WPS OfficeDocument58 pagesChemical Proces-WPS OfficeMuhammad Ahmed50% (2)
- Neil Dominic C. Mahusay Bsme-3 Module 1 Assignment: Military Body ArmorDocument4 pagesNeil Dominic C. Mahusay Bsme-3 Module 1 Assignment: Military Body ArmorMahusay Neil DominicNo ratings yet
- p608 PDFDocument8 pagesp608 PDFMohamed TabetNo ratings yet
- ME206 Lecture 3 - Key concepts in metal casting flowDocument28 pagesME206 Lecture 3 - Key concepts in metal casting flowHarsh ShahNo ratings yet
- Stabilo Ink Compliance StatementDocument2 pagesStabilo Ink Compliance StatementNurul HaziqahNo ratings yet
- Fruit Juice ClarificationDocument26 pagesFruit Juice ClarificationRoshan JainNo ratings yet
- Fan - 2009 - 8 - ECTC 2009 - 2 RahimDocument5 pagesFan - 2009 - 8 - ECTC 2009 - 2 RahimkraidonNo ratings yet
- Performance Comparative Analysis of Monocrystalline and Polycrystalline Single Diode Solar Panel Models Using The Five Parameters MethodDocument6 pagesPerformance Comparative Analysis of Monocrystalline and Polycrystalline Single Diode Solar Panel Models Using The Five Parameters MethodAlif Mu'tashimNo ratings yet
- CSR July August 2014 DigitalDocument60 pagesCSR July August 2014 Digitalolger huancara gasparaNo ratings yet
- Astm A560 A560m - 12Document3 pagesAstm A560 A560m - 12Leandro Dilkin ConsulNo ratings yet
- 27 PsychrometryDocument16 pages27 PsychrometryPRASAD326100% (1)
- 1 PDFDocument7 pages1 PDFJose Luis Martinez SaavedraNo ratings yet
- Design of Biogas DigestersDocument22 pagesDesign of Biogas DigestersSharath Chandra100% (5)
- Revised PT Program April-May 2021 30% Discount Metal TestingDocument2 pagesRevised PT Program April-May 2021 30% Discount Metal TestingSaravanan PNo ratings yet
- Feng 2020Document9 pagesFeng 2020Gerapi EraNo ratings yet
- HYDRAZINEDocument19 pagesHYDRAZINEDamla Taykoz100% (2)
- Manual For ConcretingDocument43 pagesManual For Concretinglwin_oo2435No ratings yet
- Assignment Group 13: Sodium Hydroxide Production: CH 1060 Process Engineering FundamentalsDocument61 pagesAssignment Group 13: Sodium Hydroxide Production: CH 1060 Process Engineering FundamentalsHarshil JainNo ratings yet
- WG 101Document1 pageWG 101'Lampa'No ratings yet
- Indian Railways MIG Wire Technical RequirementsDocument17 pagesIndian Railways MIG Wire Technical RequirementsJoherNo ratings yet
- Quality Tests RequirementDocument6 pagesQuality Tests RequirementSandip PaulNo ratings yet
- Exp. 4 LipidsDocument6 pagesExp. 4 LipidsAna LuisaNo ratings yet
- BCA Booklet Content Drywall Internal Partition Sept 2013Document72 pagesBCA Booklet Content Drywall Internal Partition Sept 2013Carl XhingNo ratings yet
- Items To Review at Hostel CHC ZAMKODocument1 pageItems To Review at Hostel CHC ZAMKOHumphrey OnyejegbuNo ratings yet
- Gas and Oil - ExploitationDocument12 pagesGas and Oil - ExploitationAnonymous puv25NQenNo ratings yet
- China's First Ministry of Machine-Building Standard for Radiography of WeldsDocument133 pagesChina's First Ministry of Machine-Building Standard for Radiography of Weldsmsiddique1No ratings yet
- IEC20091118133046Document5 pagesIEC20091118133046SEANMNo ratings yet
- Pet Bottles Flakes - RoughDocument19 pagesPet Bottles Flakes - RoughSivaraman P. S.100% (1)
- Accurate Self-Damage Detection by Electrically Conductive Epoxy/graphene Nanocomposite FilmDocument12 pagesAccurate Self-Damage Detection by Electrically Conductive Epoxy/graphene Nanocomposite FilmAaron ChandNo ratings yet
- Isolated Foundation Calculation (ACI 318M-95) : Input DataDocument5 pagesIsolated Foundation Calculation (ACI 318M-95) : Input DataJuan CarlosNo ratings yet